Abstract
Human endogenous retroviruses (HERVs) are ancient, currently inactive, and non-infectious due to recombination, deletions, and mutations in the host genome. However, HERV-derived elements are involved in physiological phenomena including inflammatory response. In recent studies, HERV-derived elements were involved directly in various inflammatory diseases including autoimmune diseases such as rheumatoid arthritis (RA), multiple sclerosis, amyotrophic lateral sclerosis (ALS), and Sjogren’s syndrome. Regarding the involvement of HERV-derived elements in inflammation, two possible mechanisms have been proposed. First, HERV-derived elements cause nonspecific innate immune processes. Second, HERV-derived RNA or proteins might stimulate selective signaling mechanisms. However, it is unknown how silent HERV elements are activated in the inflammatory response and what factors and signaling mechanisms are involved with HERV-derived elements. In this review, we introduce HERV-related autoimmune diseases and propose the possible action mechanisms of HERV-derived elements in the inflammatory response at the molecular level.
Retroviruses insert their genes into the host’s DNA during the viral replication process. Some retroviruses inserted into the human genome from millions of years ago are inactive and exist as endogenous retroviruses that are no longer replicated as active viruses due to various causes, such as mutations or deletions, and only traces of viral genes remain in the human genome. These endogenous retroviruses account for approximately 8 % of human chromosomal DNA. Human endogenous retroviruses (HERVs) have lost the ability to replicate and infect by forming an active virus. However, there are recent reports that viral genes of the HERV family were actively expressed and HERV-derived elements were the pathological contributor to various diseases. A typical example is the correlation between cancer and HERV elements; HERV element expression is associated with various cancers including lymphoma, breast cancer, prostate cancer,1 melanoma,2 pancreatic cancer,3 ovarian cancer,4 and leukemia.5 Based on our recently published study results, the expression of HERV-K and HERV-R Env proteins in 10 representative cancer tissues was significantly higher than in normal tissues surrounding the tumor.6 HERV elements are reportedly involved in cancer as well as other several diseases. The expression of HERV-K and HERV-W elements was significantly increased and contributed to chronic fatigue syndrome and myalgic encephalomyelitis.7
Reportedly, HERV-W plays a role in major depressive disorder and schizophrenia.7,8 Furthermore, HERV elements have been shown to be involved in autoimmune diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis, Sjogren’s syndrome, alopecia areata, type I diabetes, primary biliary cirrhosis, and systemic sclerosis.9 Inflammatory diseases accompanied by autoimmune diseases account for a significant portion of HERV-related diseases other than cancer. However, it is unclear how HERV elements affect inflammatory diseases including autoimmune disorders. In this review, we examine the relationship of HERV elements in inflammatory actions, including autoimmune diseases, and the possible involvement of HERV-derived elements underlying the mechanism of inflammatory control.
HERVs account for approximately 8 % of the human genome and play various roles; however, the contribution of HERVs to inflammatory and neurological diseases has been investigated. In a previous study, HERVs induced and increased inflammatory diseases.10 Although HERVs are involved in existing diseases, the contribution to inflammation is a newly discovered role, and the mechanism of action has yet to be established. The human body has a complex immune system for defense against invasion of external pathogenic viruses. The immune system is divided into innate immunity that responds immediately without association with a specific pathogen and acquired adaptive immunity obtained by exposure to disease or vaccination. These immune responses induce abnormal immune reactions, causing allergies or immune diseases. Excessive reactions in the immune system, such as the well-known cytokine storm, occur and attack normal cells, causing large-scale inflammatory reactions. The expression of HERV elements can trigger both innate and adaptive immunity, and immune cells can induce the expression of HERV elements. The research to date shows that abnormal activation of HERVs can induce inflammation and lead to abnormal regulation of the immune system.11 Autoimmune disease involves abnormal regulation of the HERV-related immune system.12 Autoimmune reaction is an abnormal immune response that recognizes and attacks self-antigens and includes typical autoimmune diseases such as insulin-dependent diabetes mellitus, multiple sclerosis, and RA. Typical autoimmune diseases reportedly associated with HERVs include multiple sclerosis and amyotrophic lateral sclerosis (ALS), and their relationship with HERVs is discussed in detail.
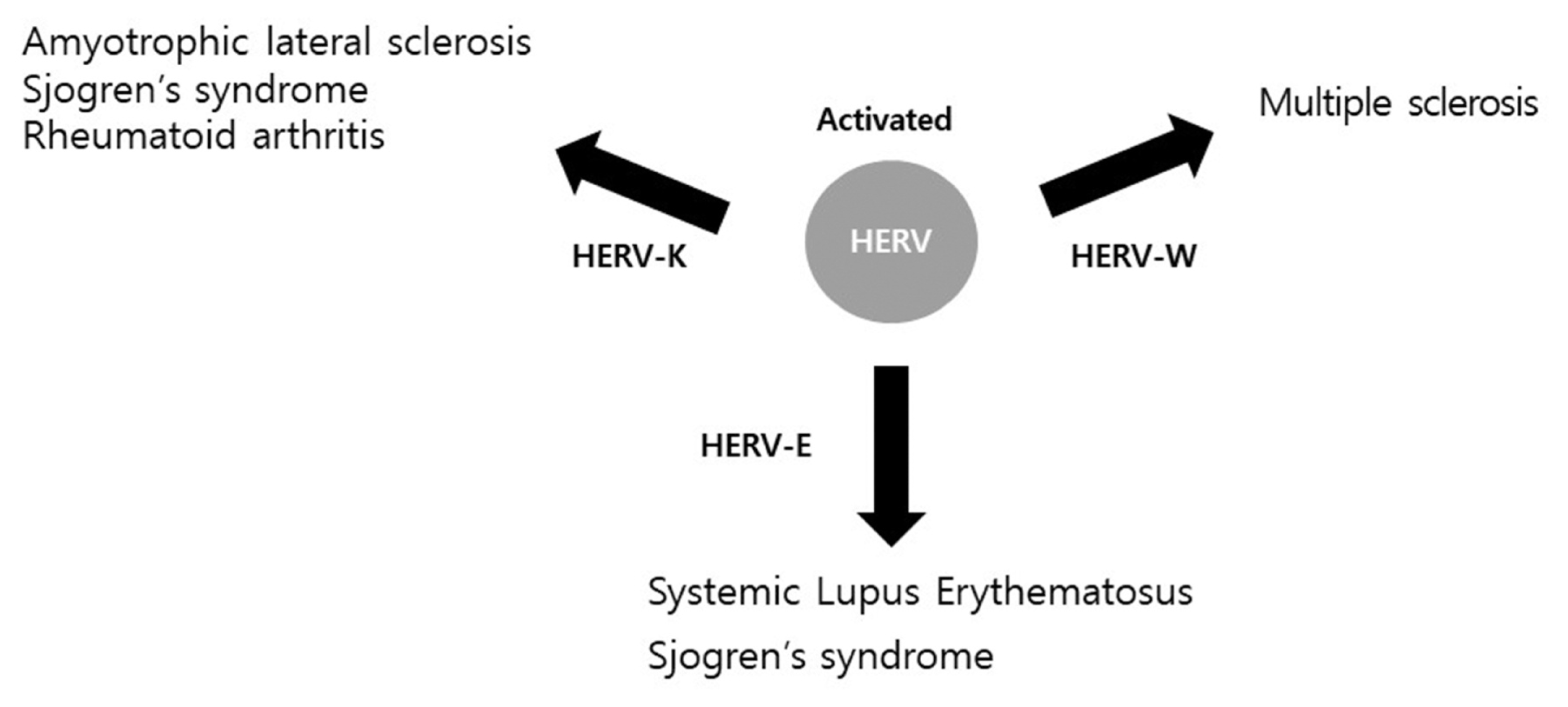
Previous study suggested that HERV elements may play an important role in initiating neurogenic inflammation.13 Multiple sclerosis is a representative neurological disease in which inflammation frequently recurs in various areas of the central nervous system. Symptoms and signs of the disease vary depending on location of pathological symptoms. The exact cause remains unknown, but autoimmune reactions play a major role in the development of multiple sclerosis in patients with genetic factors. The HERV-W family has been proposed as an important contributor to multiple sclerosis.14 The HERV-W family is activated and expression increased in neuropsychiatric and autoimmune diseases.15 Multiple sclerosis is more common in Westerners than Asians, including Koreans, and most previous studies have focused on European groups with a very high risk of multiple sclerosis. In a recent study, HERV-W expression was reported to differ depending on race and residential area.14 Another study also suggested that the expression of HERV genes as possible biomarker and target in neurodegenerative diseases including multiple sclerosis.16
ALS is a neurodegenerative disease that causes loss of muscle control by affecting nerve cells in the brain and spinal cord. HERV-K has been shown to be involved in ALS.10 Reverse transcriptase (RT) encoded by the HERV-K pol gene was detected first in the brains of ALS patients; in a follow-up study, the HERV-K gag, pol, and env genes were confirmed to be expressed in the brains of ALS patients.10,17 Expression of HERV-K elements was observed in cortical and spinal neurons of ALS patients but not in healthy individuals. Among the HERV-K elements, env, in particular, is expected to be involved in neurodegeneration. 10 Although HERV-K elements are expressed specifically in ALS, the cause of HERV-K activation in neurons in ALS patients is unknown. HERV-K activation is hypothesized to be involved in the pathogenesis of ALS, possibly through activation of an ancient fragment of HERV-K in certain areas of the central nervous system by an unknown mechanism, causing neurotoxicity. 11 In addition, HERV-K affected nerve cells in mouse experiments by reducing the length or complexity of dendrites.10 These results indicate that HERV-K is closely involved in ALS disease in various ways.
HERVs also are associated with rheumatic diseases. For example, RA is an autoimmune disease in which chronic inflammation occurs only in joints. Although the etiology of RA is unknown, HERVs have been reported as a potential cause of the disease.9 To clarify the role of HERVs in RA, serological and molecular analyses were performed, and results confirmed that HERV-K (HML-2) gag activity was significantly increased in RA patients compared with healthy individuals. 18 In addition, HERV-K mRNA was significantly upregulated in RA patients when the degree of inflammation in RA patients and control groups was compared based on real-time PCR analysis.19 Consequently, HERV-K is considered to play an important role in the onset of RA; however, the exact mechanism has not been identified, and future studies are needed to clearly analyze the association between HERV-K and RA.
Another rheumatic disease is systemic lupus erythematosus (SLE), which attacks all tissues of the body. SLE is difficult to predict, diagnose, and treat due to its various symptoms. Although the HERV activity involved in SLE remains unclear, HERV-E mRNA expression was high in CD4+T cells in SLE patients. This result indicates that HERV-E mRNA could be used as a biomarker of SLE.20 In another study, RNA-seq analysis of SLE patients and controls showed that expression of HERV LTR and sub-HERV families was significantly increased in the blood of SLE patients compared with controls.13, 21, 22 These results indicate that HERVs contribute to SLE, and further studies are needed to determine how increased expression of HERV elements affects SLE.
Sjogren’s syndrome is an autoimmune disorder in which lymphocytes invade exocrine glands such as salivary and lacrimal glands and cause chronic inflammation, leading to secretion disorders including dry mouth and eyes. The cause of Sjogren’s syndrome remains unclear, although genetic causes, viral infections, and hormonal abnormalities are expected to be involved. In a previous study, HERV-E clone 4-1 was shown to affect Sjogren’s syndrome.23 In 2005, HERV-K113 was reported to be expressed in 15.6% of 96 Sjogren’s syndrome patients in the UK, indicating HERV-K113 as a genetic risk factor for some autoimmune diseases.24 Studies about HERV in Sjogren’s syndrome in Asians are lacking.
One thing to consider to determine the correlation between HERV and inflammatory response is how the inflammatory response induces latent HERV activity. Although the mechanism by which the inflammatory response induces HERV activation has not been elucidated, several hypotheses have been proposed. The HERV locus has been identified as a potential modulator of autoimmunity,25 and changes in cytokines were suggested to induce autoimmune diseases by regulating HERV gene expression.26, 27 Various factors expressed by the inflammatory response either directly or indirectly promote HERV gene expression or activate the HERV gene that has been suppressed through epigenetic regulation. Recently, Krüppel-associated box domain zinc finger proteins (KRAB-ZFPs) were reported to regulate the expression of endogenous viruses in mice and to be involved in SLE.28 These results indicate the existence of factors regulating the expression of HERVs. Activated HERV genes are likely to contribute to immune activity at the RNA or protein level. Hypothetically, activated HERV genes induce an immune response by acting as a foreign antigen, or the RNA and proteins elicit a specific signaling mechanism to produce a selective immune response.
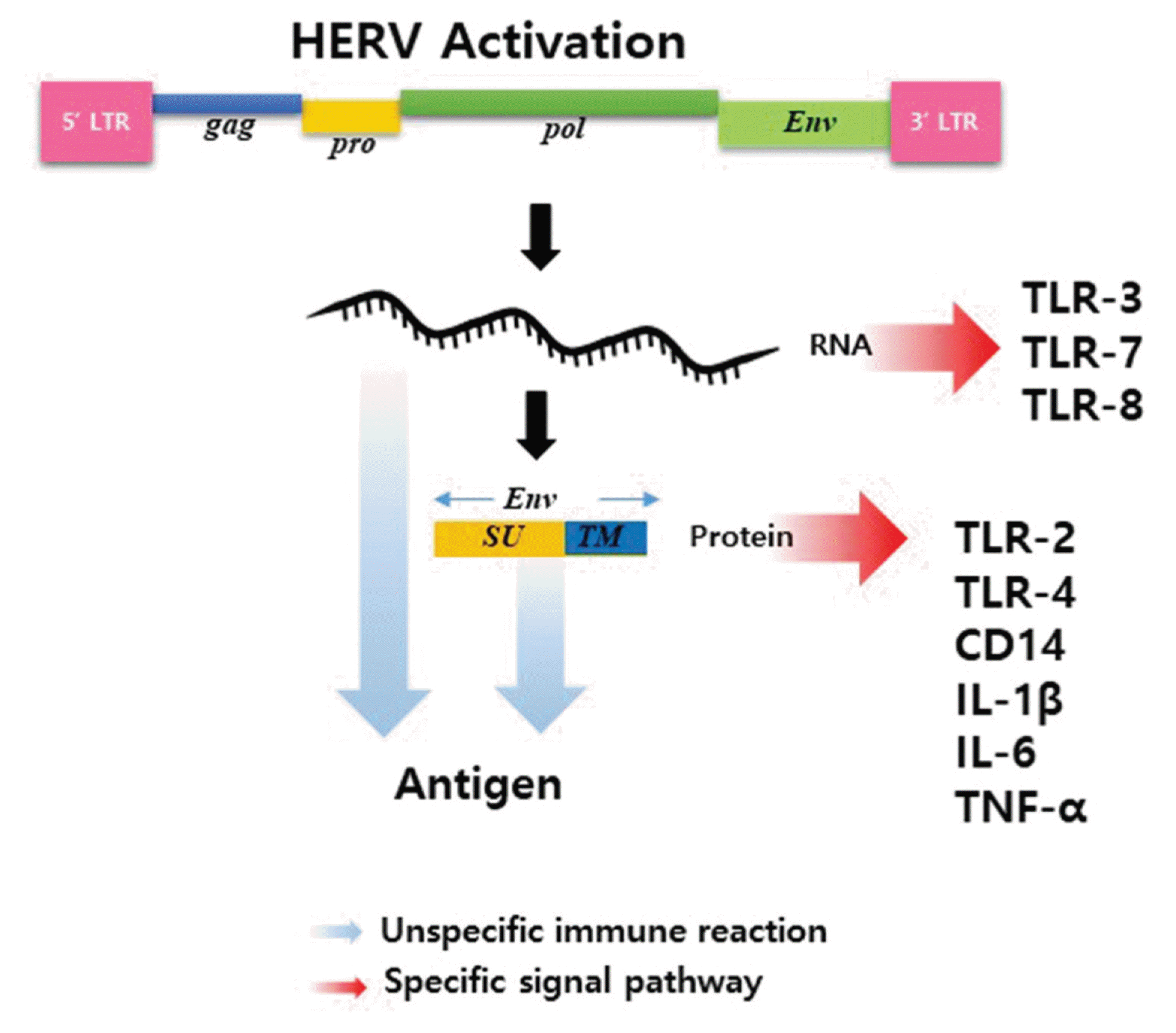
Most HERVs have an inactivated provirus genome to prevent transcription of the retrovirus gene. However, some HERVs have the potential for transcription and translation and maintain an open reading frame (ORF) within the human genome. Although expression of HERVs in healthy humans is limited, HERV gene expression might occur due to malignant transformation or epigenetic stimulation. This type of HERV gene is not normally present, but newly exists in a specific environment by certain stimulation and can be recognized as an external antigen. The HERV Env protein has been reported to induce both innate and adaptive immunity, leading to inflammation, cytotoxicity, and apoptosis.29 In addition, the HERV Env protein acts as a powerful activator of the immune system, with superantigen function, promoting nonspecific stimulation of T lymphocytes, and can lead to massive cytokine release with widespread tissue damage and systemic life-threatening symptoms (e.g., shock, multicenter failure).30 HERV-derived proteins can form antigens that can be recognized by T cells and B cells, and cell-mediated immune responses to ERV antigens have been observed in animal models and human studies. In addition, immunological self-tolerance to HERVs is incomplete. 31 In particular, HERV-W Env can induce dendritic cell maturation through the Th1-like immune response, a helper T cell, and HERV-W Env is reported to stimulate CD14/toll-like receptor 4 (TLR4) to promote the Th-1like immune response, promoting a congenital immune response.19 These results indicate that HERV-derived factors newly introduced by certain stimulation can be recognized as external antigens and cause new immune responses.
In recent studies, RNA in the HERV-K (HML-2) env gene domain was shown to bind to TLR7 in mouse neurons and microglia. The RNA also binds to human TLR8, causing neurodegeneration, indicating that HERV-K RNA participates in the neurodegenerative process by inducing a selective signaling mechanism through TLR as a ligand of TLR7 or TLR8. In another study, HERV-K env RNA induced the expression of type I interferon and cytokines by binding to MDA-5 and TLR3 receptors in THP-1 monocyte cells. These results confirmed that HERV-derived RNA is involved in a selective signaling mechanism by binding to a receptor.32 In addition, gamma irradiation triggered HERV-derived RNA binding to TLR3 to induce signal transduction in macrophages.29 These results indicate that HERV-derived RNA mediates several immune responses by binding to receptors.
Reportedly, RNA as well as HERV-derived proteins participate in the inflammatory response through a selective signaling mechanism such as direct interaction between HERV proteins and TLR. The HERV-W Env protein binds to TLR4 and CD14 and stimulates production of proinflammatory cytokines including IL-1β, IL-6, and TNF-α.19 In addition, TLR interacts with the HERV-W family and produces an inflammatory response in multiple sclerosis. TLR2 stimulated by HERV-W protein induces NF-κB to generate Th1 and Th17 cytokine responses.33, 34 Reportedly, recombinant TM protein and peptide corresponding to the immunosuppressive domain (ISD) of HERV-K inhibit the proliferation of human immune cells and regulate the release of cytokines, similar to the ISD of HIV-1.30 The SU region of the HERV Env protein has been shown to induce cell-cell fusion and fusion formation, resulting in tumorigenesis and chromosomal instability. The TM region of the protein mediates tumor cell immune evasion through immunosuppressive activity.31 These results indicate that HERV-derived proteins can play an important role in inflammatory response.
Experiments to suppress the expression of specific HERV elements using siRNA or CRISPR-Cas9 are being conducted to elucidate the functions of HERV-derived elements. Studies of siRNA treatment with HERV-K env in pancreatic cancer cell lines and removal of HERV-K env in lung cancer cell lines using the CRISPR-Cas9 system showed that tumorigenic properties including proliferation, invasion, and migration of cancer cells were significantly reduced. The HERV-K env gene was found to affect the RAS-ERK-RSK pathway in pancreatic cancer and the NUPR1-Rb pathway in colorectal cancer.3,35 Based on these study results, HERV elements regulate specific signaling pathways; however, further studies are needed to determine whether regulation of the signaling pathways occurs through RNA or proteins and what types of receptors are involved.
The reported immune diseases affected by HERV elements are summarized in Figure 1. Several HERV-derived elements have been reported to play important roles in various immune diseases, including autoimmune disorders. Although the mechanism of immunomodulatory action of HERVs has not been elucidated, promotion of various immune responses with a new antigen by activation of latent HERVs is possible, promoting multiple immune responses. Furthermore, HERV-derived RNA and proteins could combine with receptors to induce specific signaling mechanisms. The schematic diagram of the HERV mediated immune response pathway are summarized in Figure 2. However, considering the various effects and actions of HERVs, induction of new receptors and signals might be involved in addition to known mechanisms and pathways, and the specific stimuli that activate HERVs have yet to be clarified. Therefore, further research regarding HERV-derived elements is needed.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation grant funded by the Korean government (NRF-2016R1D1A3B01007444). This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A3A01086369).
REFERENCES
1. Ishida T, Obata Y, Ohara N, Matsushita H, Sato S, Uenaka A, et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008; 8:15.
2. Hahn S, Ugurel S, Hanschmann KM, Strobel H, Tondera C, Schadendorf D, et al. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res Hum Retroviruses. 2008; 24:717–23.

3. Li M, Radvanyi L, Yin B, Rycaj K, Li J, Chivukula R, et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin Cancer Res. 2017; 23:5892–911.

4. Wang-Johanning F, Rycaj K, Plummer JB, Li M, Yin B, Frerich K, et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J Natl Cancer Inst. 2012; 104:189–210.

5. Boese A, Sauter M, Galli U, Best B, Herbst H, Mayer J, et al. Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene. 2000; 19:4328–36.

6. Ko EJ, Song KS, Ock MS, Choi YH, Kim S, Kim HS, et al. Expression profiles of Human Endogenous Retrovirus (HERV)-K and HERV-R Env proteins in various cancers. BMB Rep. 2021.

7. Rodrigues LS, da Silva Nali LH, Leal COD, Sabino EC, Lacerda EM, Kingdon CC, et al. HERV-K and HERV-W transcriptional activity in myalgic encephalomyelitis/chronic fatigue syndrome. Auto Immun Highlights. 2019; 10:12.

8. Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010; 120:245–8.

9. Portis JL. Perspectives on the role of endogenous human retroviruses in autoimmune diseases. Virology. 2002; 296:1–5.

10. Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002; 82:291–329.
11. Kury P, Nath A, Creange A, Dolei A, Marche P, Gold J, et al. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol Med. 2018; 24:379–94.
12. Saleh A, Macia A, Muotri AR. Transposable Elements, Inflammation, and Neurological Disease. Front Neurol. 2019; 10:894.

13. Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science. 2015; 350:455–9.

14. Tarlinton R, Wang B, Morandi E, Gran B, Khaiboullin T, Martynova E, et al. Differential Expression of HERV-W in Peripheral Blood in Multiple Sclerosis and Healthy Patients in Two Different Ethnic Groups. Front Pharmacol. 2019; 10:1645.

15. Yu HL, Zhao ZK, Zhu F. The role of human endogenous retroviral long terminal repeat sequences in human cancer (Review). Int J Mol Med. 2013; 32:755–62.

16. Dolei A, Ibba G, Piu C, Serra C. Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases. Int J Mol Sci. 2019; 20.

17. Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011; 69:141–51.

18. Freimanis G, Hooley P, Ejtehadi HD, Ali HA, Veitch A, Rylance PB, et al. A role for human endogenous retrovirus-K (HML-2) in rheumatoid arthritis: investigating mechanisms of pathogenesis. Clin Exp Immunol. 2010; 160:340–7.

19. Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006; 176:7636–44.

20. Wu Z, Mei X, Zhao D, Sun Y, Song J, Pan W, et al. DNA methylation modulates HERV-E expression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci. 2015; 77:110–6.

22. Groger V, Cynis H. Human Endogenous Retroviruses and Their Putative Role in the Development of Autoimmune Disorders Such as Multiple Sclerosis. Front Microbiol. 2018; 9:265.

23. Hishikawa T, Ogasawara H, Kaneko H, Shirasawa T, Matsuura Y, Sekigawa I, et al. Detection of antibodies to a recombinant gag protein derived from human endogenous retrovirus clone 4-1 in autoimmune diseases. Viral Immunol. 1997; 10:137–47.

24. Moyes DL, Martin A, Sawcer S, Temperton N, Worthington J, Griffiths DJ, et al. The distribution of the endogenous retroviruses HERV-K113 and HERV-K115 in health and disease. Genomics. 2005; 86:337–41.

25. Laska MJ, Troldborg A, Hauge EM, Bahrami S, Stengaard-Pedersen K. Human Endogenous Retroviral Genetic Element With Immunosuppressive Activity in Both Human Autoimmune Diseases and Experimental Arthritis. Arthritis Rheumatol. 2017; 69:398–409.

26. Stauffer Y, Marguerat S, Meylan F, Ucla C, Sutkowski N, Huber B, et al. Interferon-alpha-induced endogenous superantigen. a model linking environment and autoimmunity. Immunity. 2001; 15:591–601.
27. Clerici M, Fusi ML, Caputo D, Guerini FR, Trabattoni D, Salvaggio A, et al. Immune responses to antigens of human endogenous retroviruses in patients with acute or stable multiple sclerosis. J Neuroimmunol. 1999; 99:173–82.

28. Treger RS, Pope SD, Kong Y, Tokuyama M, Taura M, Iwasaki A. The Lupus Susceptibility Locus Sgp3 Encodes the Suppressor of Endogenous Retrovirus Expression SNERV. Immunity. 2019; 50:334–47.e9.

29. Mikhalkevich N, O’Carroll IP, Tkavc R, Lund K, Sukumar G, Dalgard CL, et al. Response of human macrophages to gamma radiation is mediated via expression of endogenous retroviruses. PLoS Pathog. 2021; 17:e1009305.

30. Morozov VA, Morozov AV, Semaan M, Denner J. Single mutations in the trans-membrane envelope protein abrogate the immunosuppressive property of HIV-1. Retrovirology. 2012; 9:67.

31. Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun. 2004; 72:3129–37.
32. Dembny P, Newman AG, Singh M, Hinz M, Szczepek M, Kruger C, et al. Human endogenous retrovirus HERV-K(HML-2) RNA causes neurodegeneration through Toll-like receptors. JCI Insight. 2020; 5.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download