Abstract
The etiology of the vertebral artery dissecting aneurysm (VADA) is unknown and they frequently occur in relatively healthy young men. Therefore, the pathological mechanism by which VADAs occur has not been accurately identified. In this paper, we will examine a case in which a young man complaining of a simple headache became unconscious due to the rupture of a VADA in grew immediately.
The first vertebral angiographic and direct surgical observation of such an aneurysm was made by Yonas et al. (1977)1; earlier case reports were all pathological studies.2 However, the etiology of spontaneous vertebral artery dissection remains unknown because it frequently occurs in relatively young men without atherosclerotic factors.3–4 Therefore, the pathogenesis of the dissection remains obscure.5 The mean age of occurrence was reported in the range 35–53 years.1 In general, spontaneous vertebral artery dissection occurs as blood vessels dissect over several months and years.6
We report a patient with subarachnoid hemorrhage due to the rupture of a VADA, who lost consciousness in only one day after complaining of a headache.
A 55-year-old male patient who visited the emergency room of our hospital due to decreased consciousness was confused at the time of his visit, and his pupil reflex was fixed at 5 mm on the right and left. Brain computed tomography (CT) taken after visiting the hospital showed hydrocephaly with subarachnoid hemorrhage (Fig. 1). In addition, on contrast-enhanced CT angiography, spontaneous dissecting aneurysm was observed in the left vertebral artery (Fig. 2).
The patient developed a headache two days before visiting the emergency room of our hospital and received medication after being admitted to another medical institution. After that, there was no improvement in symptoms, so brain magnetic resonance imaging (MRI) was taken and no abnormalities were found in the image. No hemorrhage was found in the susceptibility weighted imaging of MRI, and the left vertebral artery was observed as normal in the magnetic resonance angiography (Fig. 3). The dissection of the patient’s vertebral artery took only two days.
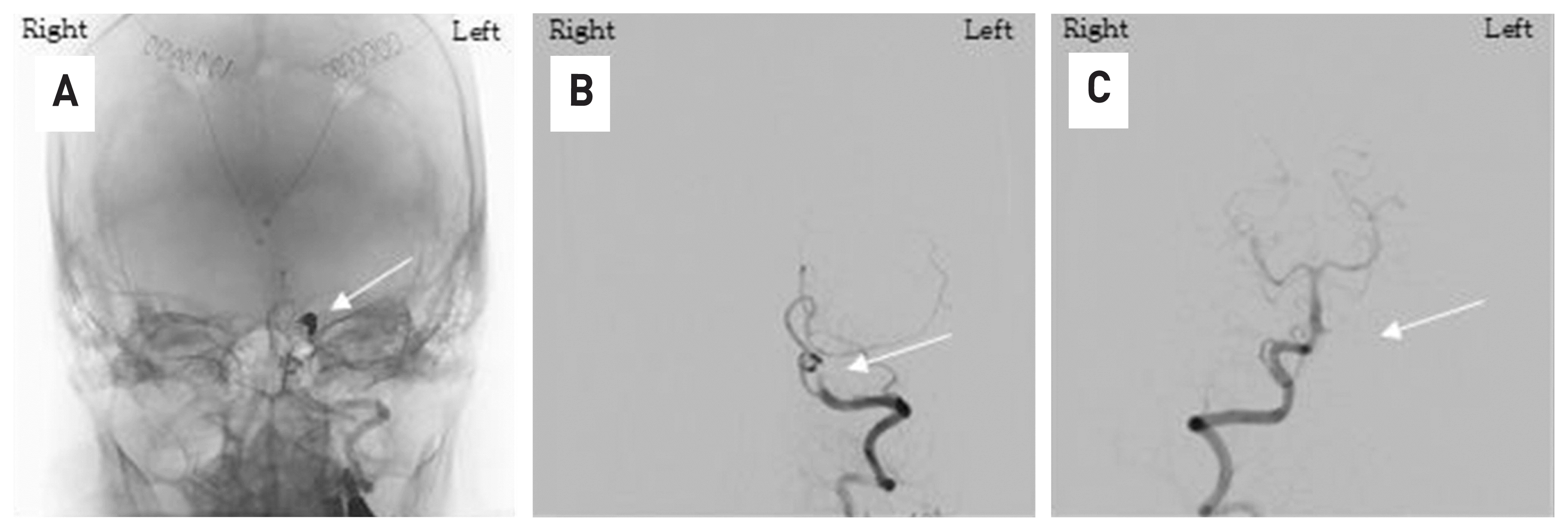
At the time of his arrival at our hospital, the patient was unconscious and the guardian was given the patient’s medical history. It was judged that this was the cause of the cerebral hemorrhage and the treatment of the patient’s increased intracranial pressure was considered to be a priority, so external ventricular drain surgery was performed first as an emergency. Immediately after surgery, transfemoral cerebral angiography was performed in the angiography room and a dissecting aneurysm of the left vertebral artery could be confirmed (Fig. 4). We thought that range of the dissected vertebral artery was long and the posterior inferior cerebellar artery was located at the proximal part of the lesion, so trapping the vertebral artery with coil embolization was possible. The procedure finished safely and complete obstruction of the lesion could be observed in the last cerebral angiography (Fig. 5).
The patient is still treated in the intensive care unit without recovering consciousness, although neurological symptoms have not worsened after the procedure.
Spontaneous intracranial vertebral artery dissection exhibits various clinical symptoms in young adults. The neurological symptom such as headache, ischemic stroke, and subarachnoid hemorrhage are exhibited.7,8
The clinical symptoms, pathology, epidemiology, and prognosis of VADA have been described in many papers.2–4 The exact rate of occurrence of dissecting vertebral artery aneurysms with subarachnoid hemorrhage is unknown; however, this condition is clearly not rare. Yamaura et al.9 reported that 24 (28%) of their 86 patients with vertebral artery aneurysms had dissecting aneurysms; 21 of these patients presented with subarachnoid hemorrhage and the other three with ischemia. In the present series,9,10 which covered a period of more than three years, 20 (42%) of the 48 vertebrobasilar ruptured aneurysms treated were of the dissecting vertebral artery type, constituting 10% of all cases defined by angiography in this period.
However, only a few papers11,12 have reported on VADA that develop very rapidly as in this case. In this case, the patient expressed symptoms of headache, took a brain MRI, and a subarachnoid hemorrhage occurred due to vertebral artery dissection a next day. Inui Y et al.10 described a ruptured vertebral dissecting aneurysm on the opposite side that occurred after trapping the contralateral vertebral artery. Although it is similar to our paper in that it was a rapidly occurring dissecting aneurysm, that study described a clear reason for the occurrence of a ruptured dissecting aneurysm.
It is thought that the normal vertebral artery was subjected to hemodynamic stress due to the trapping of the opposite vertebral artery to develop dissection; however, in our paper, there were no factors that caused any hemodynamic stress. The only hemodynamic stress that can be suspected was an increase in blood pressure due to headaches, and in fact, the patient’s systolic blood pressure at other hospitals was high at around 150. This is a valid hypothesis, but it is difficult to find any accurate mechanism due to the dissecting.
There are two main treatments for VADA: surgical treatment and endovascular treatment. There are advantages and disadvantages to each treatment method. First of all, surgical treatment methods have the advantage of low recurrence, but there are difficulties and risks in accessing lesions.13,14 Endovascular embolization is the preferred strategy for VADA due to its safety and efficiency. The methods of endovascular treatment are divided into two main methods: reconstruction and deconstruction. Destruction technique refers to parent artery occlusion. The reconstruction technique consists of stent implantation with or without coiling and flow–diverting stent.15,16 In our patient, posterior inferior cerebellar artery was located in the proximal part of the lesion, and the blood flow of the opposite vertebral artery was good, so trapping using coil, a construction method, was attempted, and the results were good. No complications such as rebleeding or infarction occurred for a month after the procedure. In the case of other patients diagnosed with ruptured VADA, most of them were diagnosed with VADA on the previous brain image, became ruptured during follow-up, or After rupture, patients visited the hospital and was diagnosed for the first time. In this case, we were able to identify a ruptured VADA within 24 hours of very rapid development. Based on this case of evidence, careful follow-up is also required for non-specific headache patients who deviate from the typical headache pattern(severe headache in occipital area, neck pain, stroke, loss of consciousness) of VADA patients.17
REFERENCES
1. Yonas H, Agamanolis D, Takaoka Y, White RJ. Dissecting intracranial aneurysms. Surg Neurol. 1977; 8:407–15.
2. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; 36:905–13.

3. Adams HP Jr, Aschenbrener CA, Kassell NF, Ansbacher L, Cornell SH. Intracranial hemorrhage produced by spontaneous dissecting intracranial aneurysm. Arch Neurol. 1982; 39:773–5.

4. Alexander CB, Burger PC, Goree JA. Dissecting aneurysms of the basilar artery in 2 patients. Stroke. 1979; 10:294–9.

5. Brown OL, Armitage JL. Spontaneous dissecting aneurysms of the cervical internal carotid artery. Two case reports and a survey of the literature. Am J Roentgenol Radium Ther Nucl Med Actions. 1973; 118:648–53.
6. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurgery. 2001; 94:712–7.

7. Hosoya T, Adachi M, Yamaguchi K, Haku T, Kayama T, Kato T. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke. 1999; 30:1083–90.

8. Yamaura A, Ono J, Hirai S. Clinical picture of intracranial nontraumatic dissecting aneurysm. Neuropathology. 2000; 20:85–90.

9. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990; 72:183–8.

10. Inui Y, Oiwa Y, Terada T, Nakakita K, kamei I, Hayashi S. De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion—two case reports. Neurol Med Chir(Tokyo). 2006; 46:32–6.

11. Kubo Y, Miura K, Suzuki M, Tsuiki K, Kuwata N, Kubo N, et al. Development of a dissecting aneurysm on the vertebral artery immediately after occlusion of the contralateral vertebral artery; a case report. Neurosurg Rev. 1998; 21:177–80.

12. Naito I, Iwai T, Sasaki T. Management of intracranial vertebral aetery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002; 51:930–7.
13. Czabanka M, Ali M, Schmiedek P, Vajkoczy P, Lawton MT. Vertebral artery-posterior inferior cerebellar artery bypass using a radial artery graft for hemorrhagic dissecting vertebral artery aneurysms: Surgical technique and report of 2 cases. J Neurosurg. 2011; 114:1074–9.

14. Park W, Ahn JS, Park JC, Kwun BD, Kim CJ. Occipital artery-posterior inferior cerebellar artery bypass for the treatment of aneurysms arising from the vertebral artery and its branches. World Neurosurg. 2014; 82:714–21.

15. Su W, Gou S, Ni S, Li G, Liu Y, Zhu S, Li X. Management of ruptured and unruptured intracranial vertebral artery dissecting aneurysms. J Clin Neurosci. 2011; 18:1639–44.

Fig. 1
In A, a subarachnoid hemorrhage with high signal is observed along the sylvian fissure. In B, because of SAH, hydrocephalus is observed.
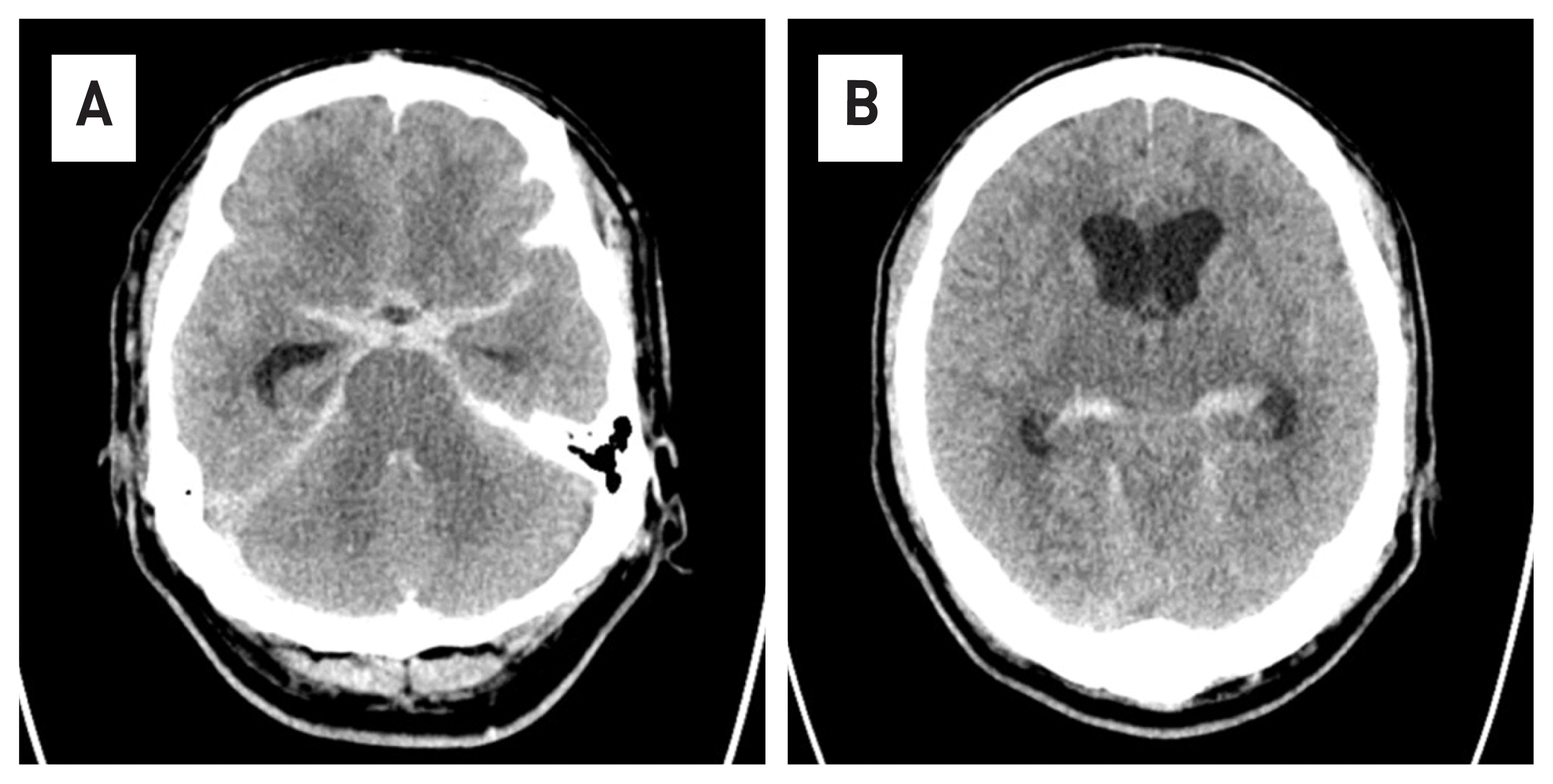
Fig. 2
A spontaneous dissecting with vascular dilatation and stenosis is observed in the left vertebral artery. (white circle)
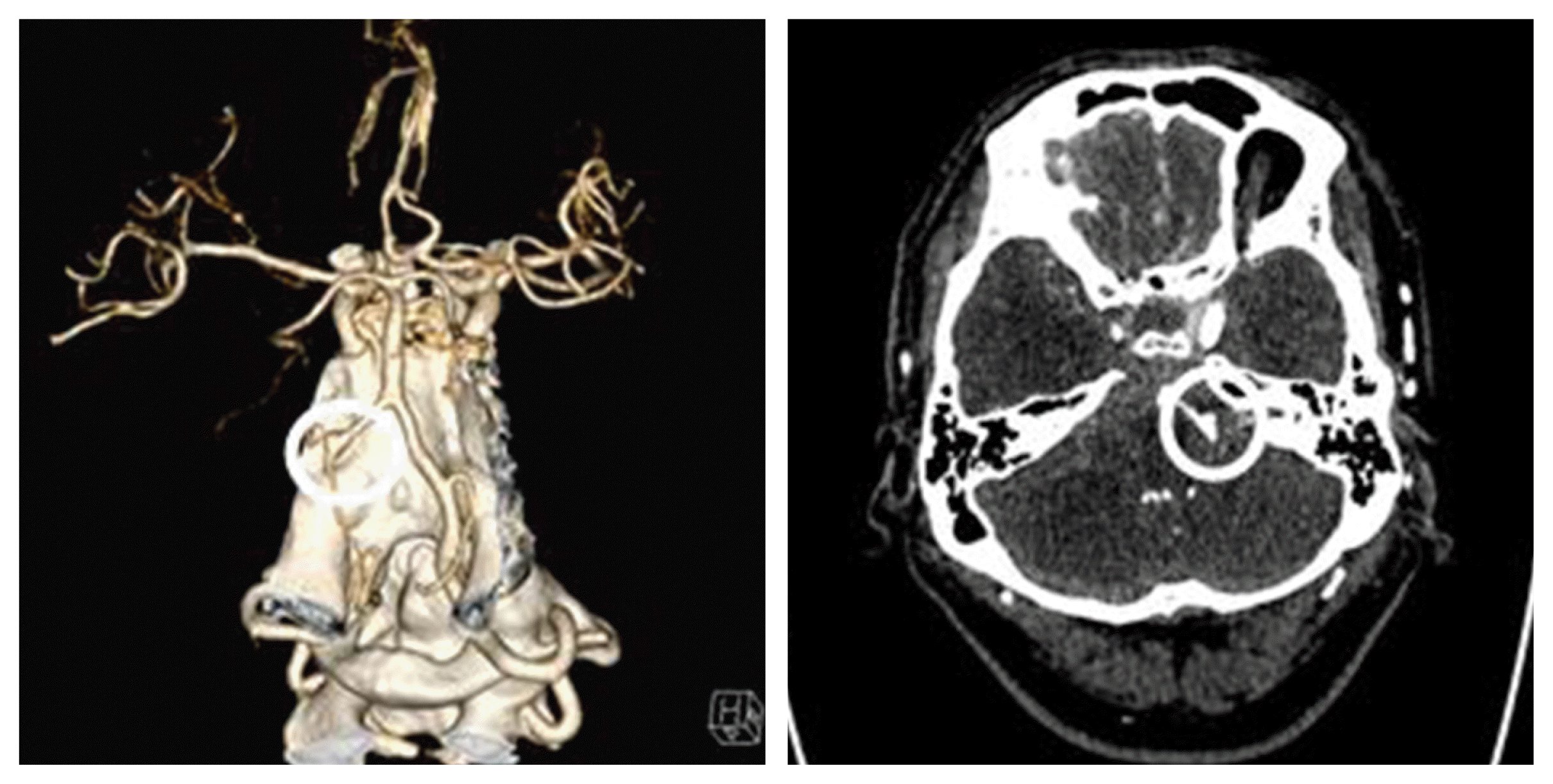
Fig. 3
No bleeding was observed in the susceptibility weighted imaging of brain MRI and normal findings were found (A,B). In the angiography of brain MRI, no dissection of the vertebral artery observed (white circle)(B).
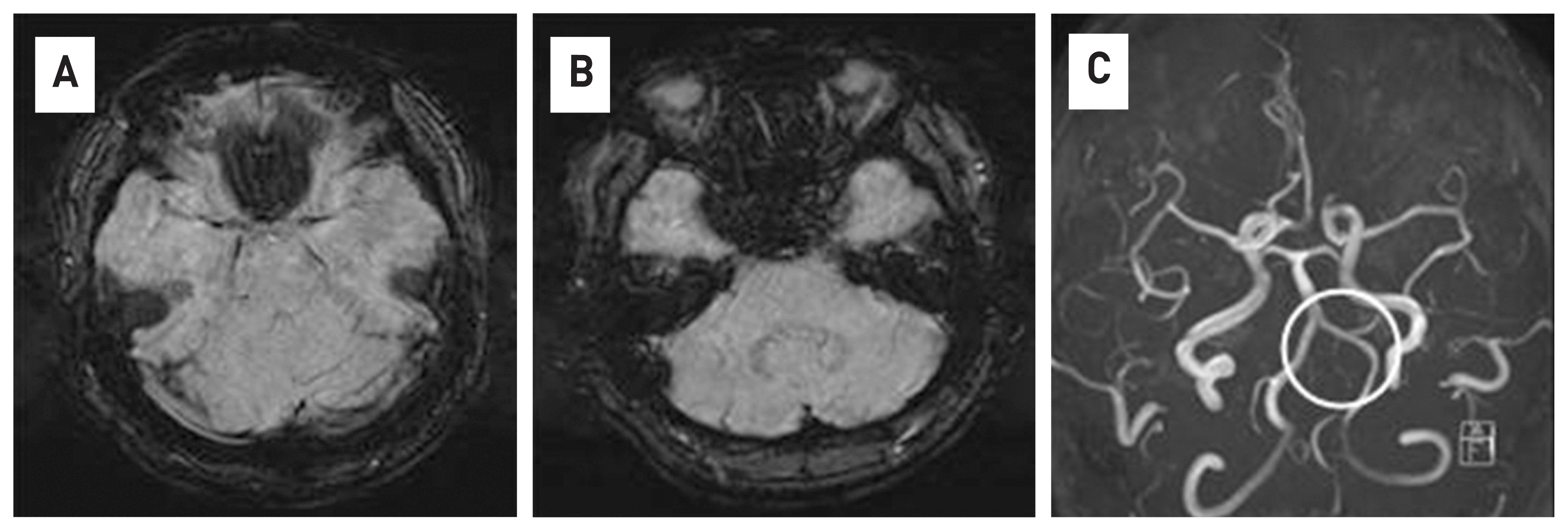
Fig. 4
In cerebrovascular angiography, spontaneous dissecting of the left vertebral artery with contrast leakage is observed (white arrow). (A) anterior-posterior view, (B) lateral view.
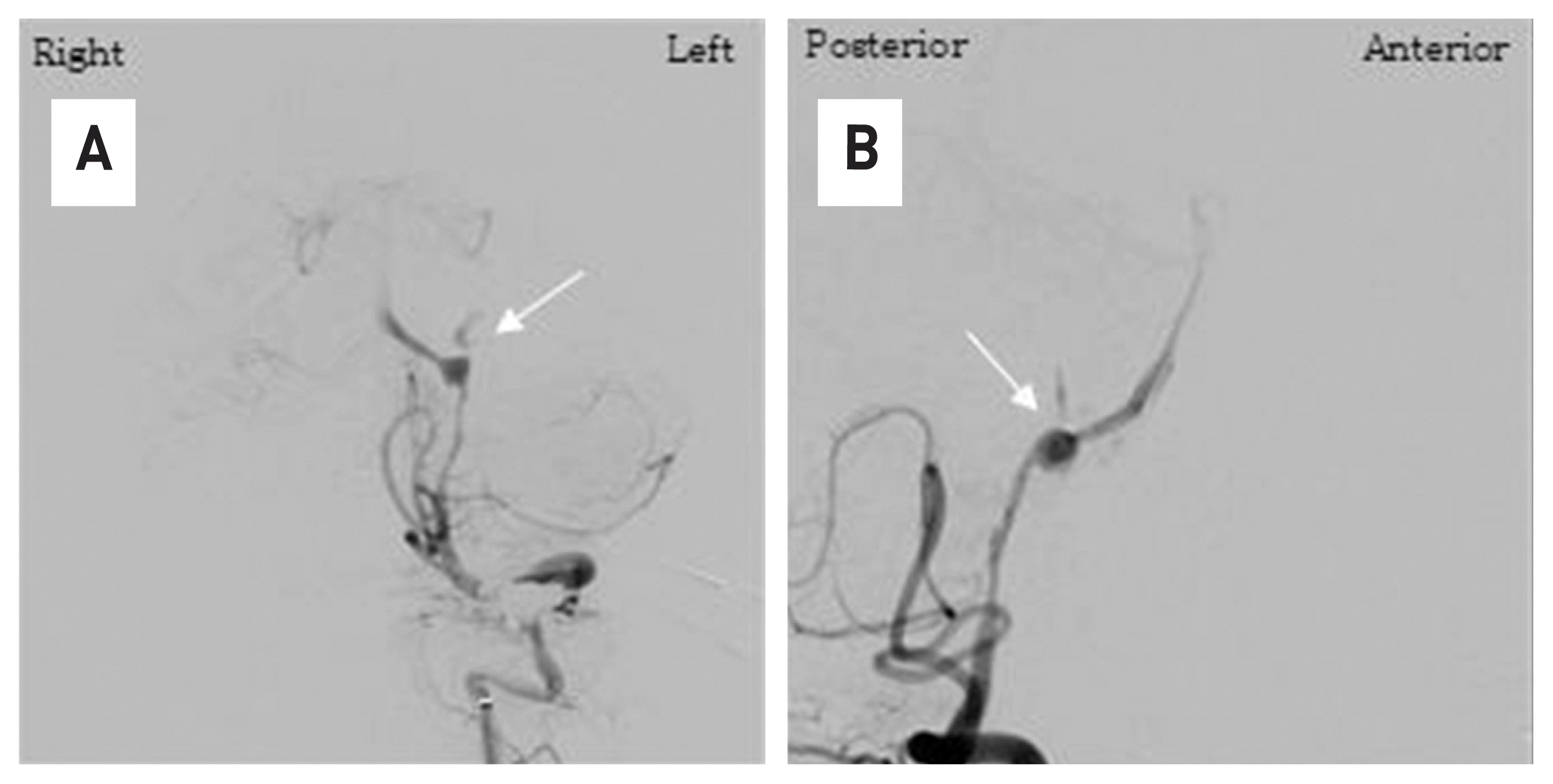
Fig. 5
With the coil mass that occluded the aneurysm sac (A, white arrow), cerebral angiography of the left vertebral artery shows complete obstruction of the lesion (B, white arrow). Angiography of the opposite vertebral artery confirmed that there was no blood flow into the lesion retrograde (C, white arrow) (A,B,C: anterior-posterior view).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download