Abstract
The flow diverter device (FDD) is an important treatment method for cerebral aneurysms, especially for intracranial dissecting aneurysms. This paper is the result of FDD treatment for two cases of vertebral dissecting aneurysm (VADA) patients and short-term follow-up at 3 months. All two cases were targeted for unruptured cerebral aneurysm, and 4-vessel angiography was performed as a follow-up examination for 3 months after receiving the procedure. As result, it was possible to shorten the period of use of antiplatelet drugs.
In the case of VADA, there are limitations in general coiling procedures or conventional surgical treatment methods. In that sense, the FDD treatment method can be a very effective alternative treatment of VADA
Despite the recent development in the endovascular treatment area of intracranial aneurysms, there are clear limitations in the treatment of large (15–25 mm) and giant (> 25 mm) aneurysms. The Flow diverter devices (FDD) are important tools in the treatment of large and giant intracranial aneurysms. FDD exhibit low rates of complication while possessing high occlusion rates in the treatment of these intracranial aneurysms.1–3
These new devices consist of a high-attenuation braided mesh stent placed in the parent artery at the level of the neck to disrupt the intra-aneurysmal flow and subsequently create intra-aneurysmal thrombosis; the exposed surface of the FDD also provides good support for the development of the neointima.4 Unlike previous conventional coiling treatments, these FDDs gradually slow down the flow of blood to the aneurysm sac through the blood flow diversion effect, and finally form an aneurysm occlusion in the formation of a thrombus and neointima.5–9 In most papers, the effectiveness of FDD is confirmed through more than 1 year of follow-up observation. 5–7 However, the longer the follow-up period, the longer the patient’s used anticoagulants. Therefore, in this paper, I would like to present two cases that set the follow-up period to 3 months to reduce the patient’s anticoagulation time.
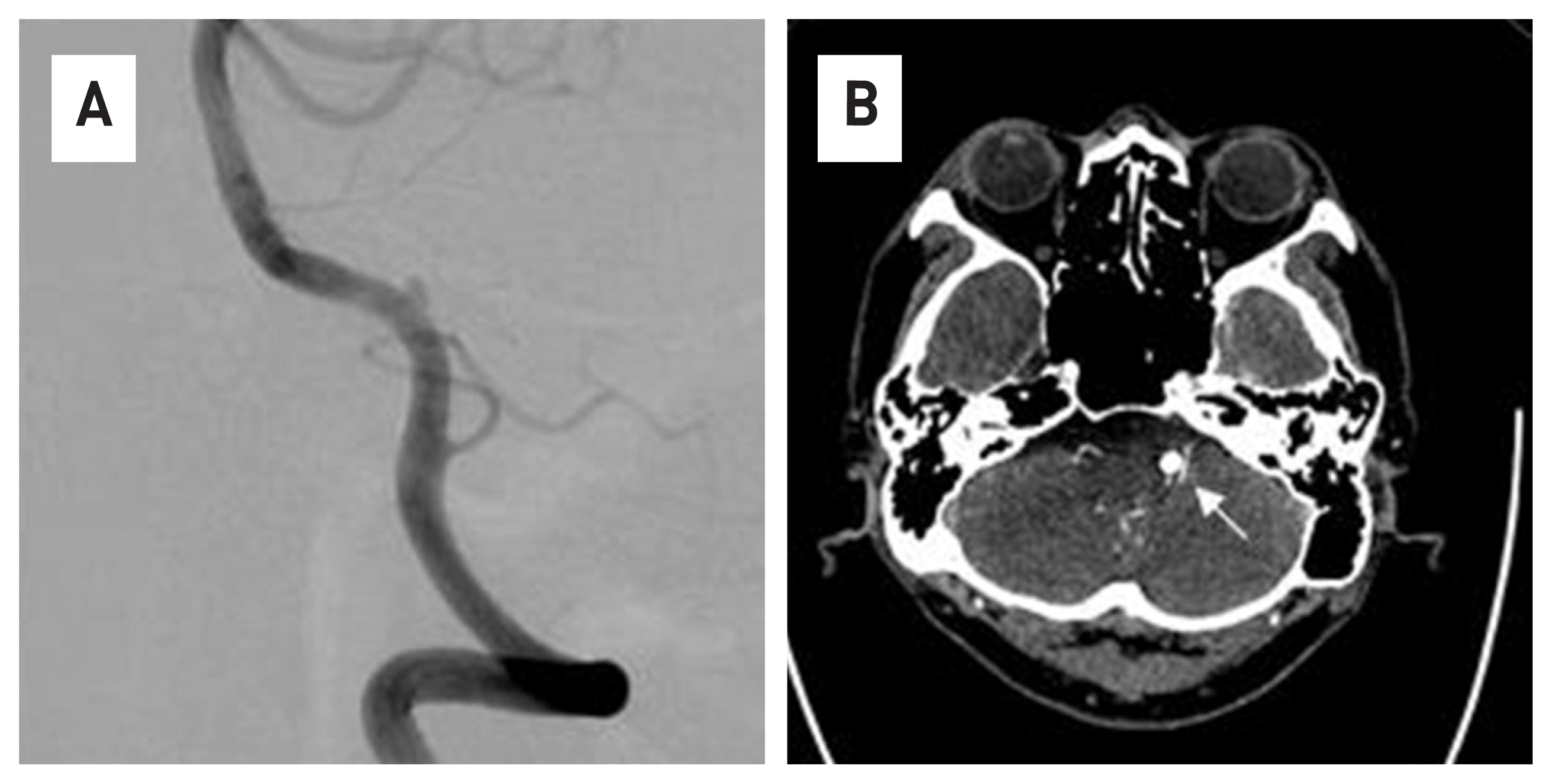
63-year-old male patient who visited the hospital due to persistent occipital-area headache and dizziness. A vertebral dissection aneurysm (VADA) was found in the right vertebral artery in a brain magnetic resonance image (MRI) and 4-vessel angiography (Fig. 1). The length and width of the VADA were 14.50 mm × 7.82 mm. (Fig. 1B) After taking dual anticoagulants for 2 weeks, the FDD procedure was performed under general anesthesia. The operation took about an hour. Through the right vertebral artery, under the guide of Synchro® 14 microwire, Excelsior ® XT-27® Microcatheter is located in the distal basilar artery. After loading Flex Embolization Device with Shield Technology™ - PED2-350-30 on Excelsior® XT-27® Microcatheter, stent was deployed through pull and push technique (Fig. 2). The patient was discharged without neurological complications, and a follow-up examination was performed at 3 months later. In the 4-vessel image taken 3 months later, the cerebral aneurysm was complete occlusion, and all flow of the parent artery were intact (Fig. 3A). In addition, in the source image of Brain CT, recovery to the normal vascular size is observed in the right vertebral artery where the lesion was observed (Fig. 3B).
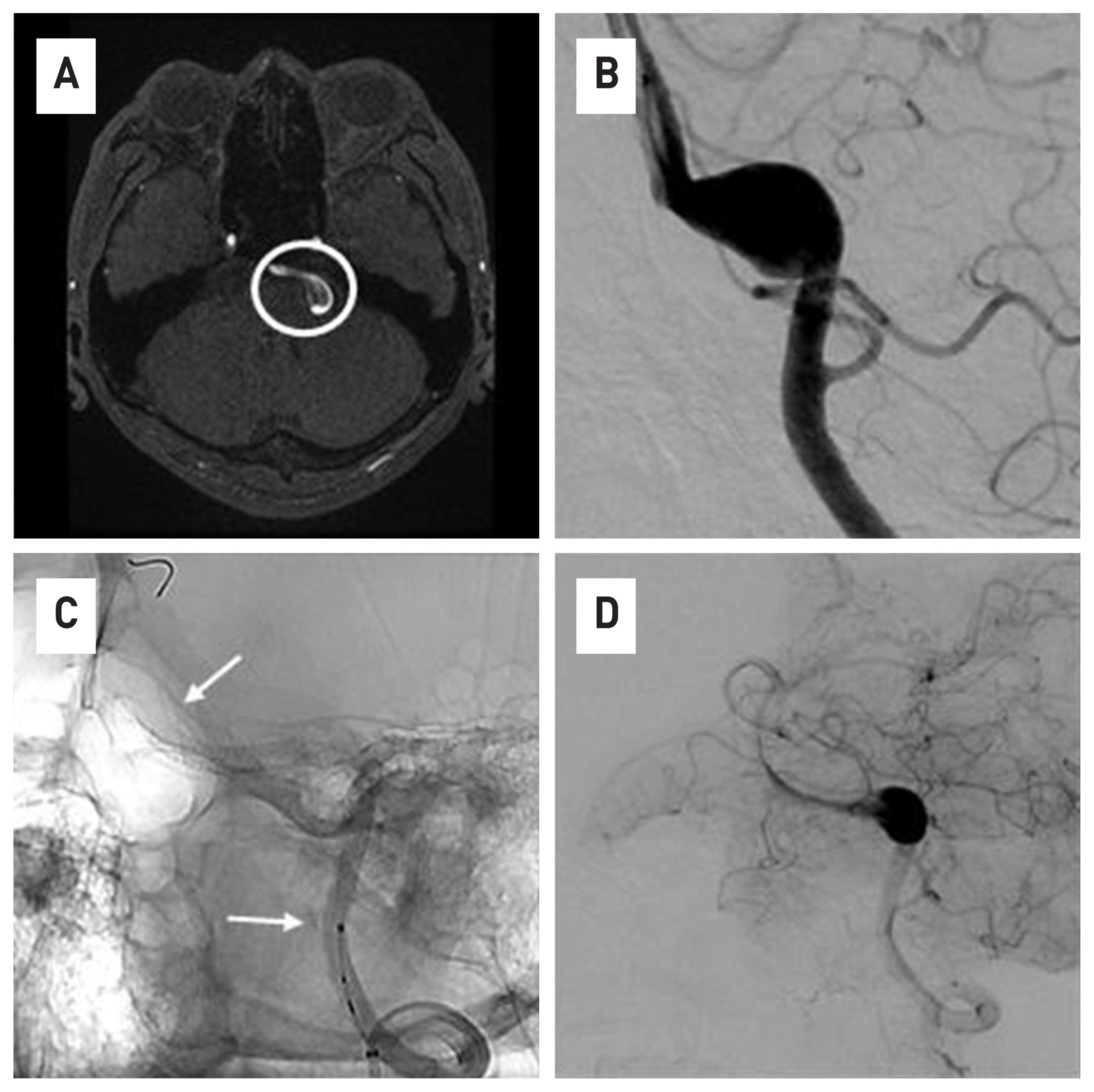
The second patient was a 47-year-old male patient who visited the emergency room with headache, dysarthria, and weakness of the right upper limb. The VADA was observed in the left vertebral artery on brain MRI and 4-vessel angiography, and the brainstem was being compressed due to mass effect (Fig. 4A). And The length and width of the VADA were 14.70 mm × 9.37mm (Fig. 4B). The operator was the same person as the previous case, and the operation took about an hour. Through the left vertebral artery, the Excelsior® XT-27® Microcatheter was placed under the guide of Synchro® 14 microwire, navigation to the distal portion of the basilar artery. After loading Flex Embolization Device with Shield Technology™-425-35 into the Excelsior® XT-27® Microcatheter, pull and push were repeated to deploy Flex Embolization Device with Shield Technology™-425-35 (Fig. 4C, D). The procedure was performed without problems. In the case of this patient, follow-up tests were performed for 3 months, and all neurological symptoms had disappeared at the time of the work up. VADA disappeared from the 4-vessel image taken 3 months later, and the lesion site had a normal course of vertebral artery (Fig. 5).
Unlike conventional neuro-interventional therapy, the treatment purpose of FDD is to delay and slow the rate and amount of blood flowing into the intracranial cerebral aneurysm, resulting in thrombosis and an expected obstruction of the aneurysm and preservation of the patency of the adjacent vessels. Unlike other stents settled on the vessel wall by radial force, when stent is deployed after loading on the microcatheter, FDD has a weak radial force, which causes technical difficulties during the procedure. Using the tension of pull and push appropriately, the operator should attach FDD directly to the vessel wall, especially when deploying past the entrance of the aneurysm, appropriate force should be applied to prevent FDD from being herniation toward the aneurysm. When FDD is herniated inside the aneurysm sac, the flow diversion effect disappears, and the procedure fails.
In 2013, Brinjikji et al. reported a meta-analysis of 29 studies representing 1451 patients treated with FDD and found complete occlusion, morbidity, and mortality rates of 76%, 5%, and 4%, respectively.10 Morbidity and mortality reported in this paper were primarily caused by cerebral infarction. As such, FDD is prone to complications caused by cerebral infarction, so antiplatelet agents must be taken before surgery. A typical protocol would include aspirin (81–325 mg) and clopidogrel (75 mg) for 5–7 days pre-procedure, with or without a loading dose of clopidogrel. The post-procedural duration of dual antiplatelet drugs also is not standardized. Generally, clopidogrel (75 mg) is continued for 6 months and aspirin (81–325 mg) is continued for a minimum of 6 months.11 As such, the long duration of antiplatelet drugs is a disadvantage of FDD treatment; thus, recently, short term follow-up has been studied to reduce this period.
The pipeline for uncoilable or failed aneurysms (PUFS) trial is perhaps the best-known. PUFS included 180-day follow up of patients undergoing FDD deployment. The trial prospectively enrolled 108 patients over an 8-month period at 10 United States medical centers. FDDs were successfully deployed in 99.1% of patients. Monotherapy with the FDD resulted in complete occlusion in 73.8%, 86.8%, and 92.1% at 6-, 12-, and 36-month angiographic follow-up visits, respectively, with a 5.6% showing 180-day major complications.12
Although the probability of cerebral aneurysm occlusion increases over time, it can be seen that the closing rate of 73.8% for 6 months is also very high. In the two cases conducted in this study, follow-up observation was performed at 3 months, and as a result, the cerebral aneurysms were completely occluded. Additionally, the formation of neointima was complete, and flow stagnation was not observed. In addition, several papers have reported that FDD forms neointima in blood vessels faster than other stents used in cerebral vessel.13–15
Unlike other stents used in cerebral vessel, in the case of FDD, if the stent cell is not fully attached to the vessel wall, the flow diversion effect of the part decreases, and the stagnation of contrast is observed in 4-vessel angiography. And if the neointima of the FDD is not formed, blood flow is formed into parts, and as a result, the stagnation of contrast is observed in the 4-vessel angiography. In this case, thrombo-embolic events occur at any time, so the use of antithrombotic agents is required.
Based on these results, we stopped antiplatelet drugs after three months, and the patients showed no complications after that. The first case has been undergoing follow-up for 20 months after the procedure, and the second case for 2 years, no complications have been observed. Endovascular treatment with the FDD is a safe and effective treatment option for intracranial aneurysms and is associated with a high occlusion rate and low risk of complications. The use of antiplatelet drugs, which must be taken for a long time after surgery, is considered a disadvantage. However, in recent studies, short follow-up observations are gradually reducing the use of antiplatelet agents.15–17 In this study, a very short follow-up period of 3 months confirmed complete occlusion of the cerebral aneurysms. Finally, more studies are needed before drawing firm conclusions, and it seems necessary to continuously observe the patient’s progress. Conclusions should be drawn through continuous research of patients with the same disease.
REFERENCES
1. Brinjikji W, Murad HM, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–7.

2. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. 2015; 28:365–75.

3. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013; 73:193–9. discussion 199–200.
4. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 38:2346–52.

5. Maimon S, Gonen L, Nossek E, Strauss I, Levite R, Ram Z. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien). 2012; 154:979–87.

6. Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, et al. Flow-diverter Silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012; 33:1150–5.

7. Wagner A, Cortsen M, Hauerberg J, Romner B, Wagner MP. Treatment of intracranial aneurysms. Reconstruction of the parent artery with flow-diverting (Silk) stent. Neuroradiology. 2012; 54:709–18.

8. Mpotsaris A, Skalej M, Beuing O, Eckert B, Behme D, Weber W. Long-term occlusion results with SILK flow diversion in 28 aneurysms: do recanalizations occur during follow-up? Interv Neuroradiol. 2015; 21:300–10.

9. Shankar JJ, Tampieri D, Iancu D, Cortes M, Agid R, Krings T, et al. SILK flow diverter for complex intracranial aneurysms: a Canadian registry. J Neurointerv Surg. 2016; 8:273–8.

10. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–7.

11. Texakalidis P, Bekelis K, Atallah E, Tjoumakaris S, Rosenwasser RH, Jabbour P. Flow diversion with the pipeline embolization device for patients with intracranial aneurysms and antiplatelet therapy: a systematic literature review. Clin Neurol Neurosurg. 2017; 161:78–87.

12. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013; 267:858–68.

13. Matsuda Y, Jang DK, Chung JH, Wainwright JM, Lopes D. Preliminary outcomes of single antiplatelet therapy for surface-modified flow diverters in an animal model: analysis of neointimal development and thrombus formation using OCT. J NeuroIntervent Surg. 2019; 11:74–9.

14. Marosfoi M, Clarencon F, Langan ET, King RM, Brooks OW, Tamura T, et al. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. 2018; 10:406–11.

15. Hagen MW, Girdhar G, Wainwright J, Hinds MT. Thrombogenicity of flow diverters in an exvivo shunt model: effect of phosphorylcholine surface modification. J Neurointerv Surg. 2017; 9:1006–11.
Fig. 1
In the brain MRI(A) and 4V angiography(B), a large dissecting aneurysm is observed in the right vertebral artery.
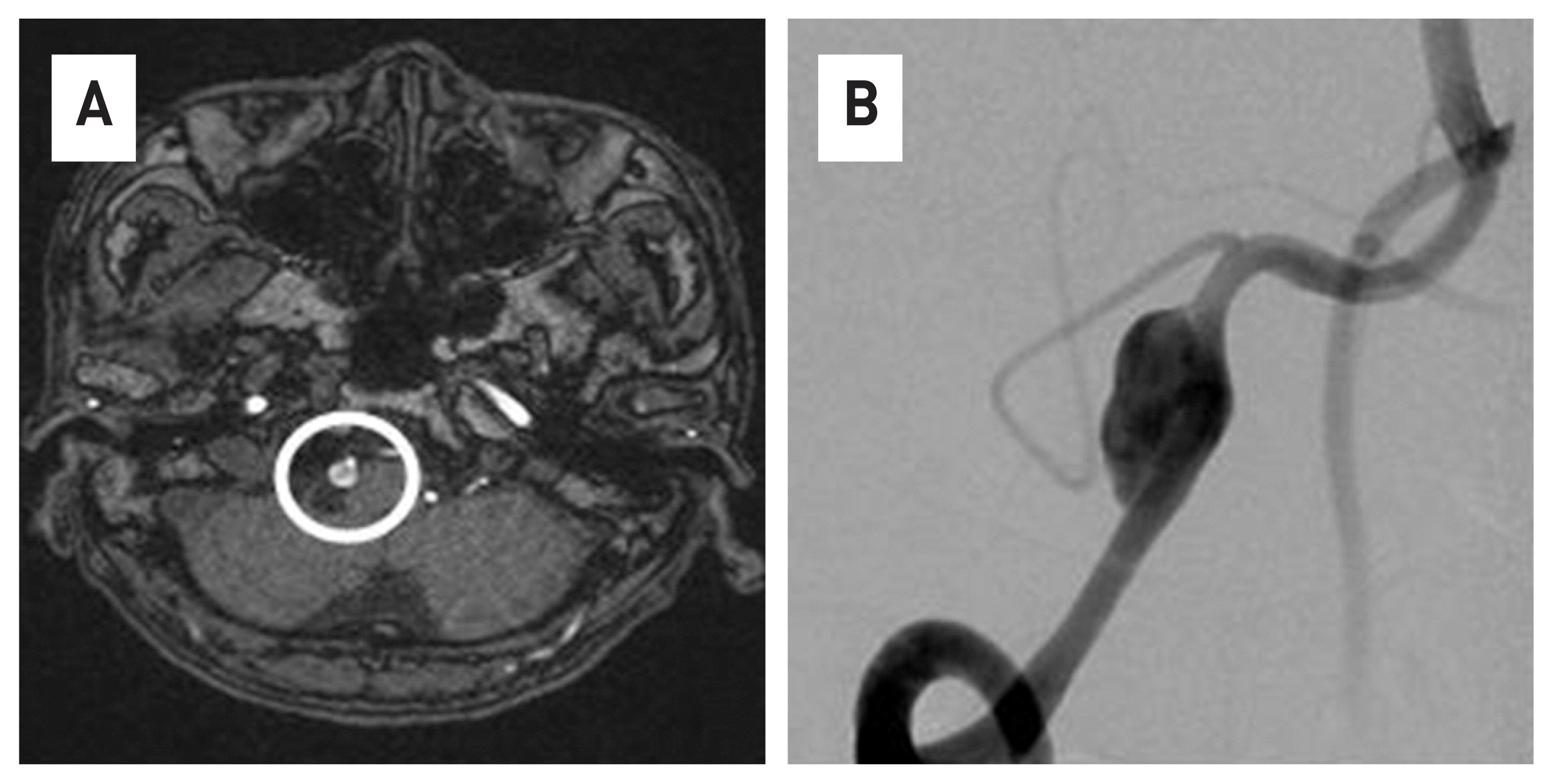
Fig. 2
After placing the microscatheter at the distal part of the right vertebral artery, FDD was loaded into the microscatheter. (A) Then, the microcatheter was removed and FDD was deployed in the lesion(black arrow)(B).
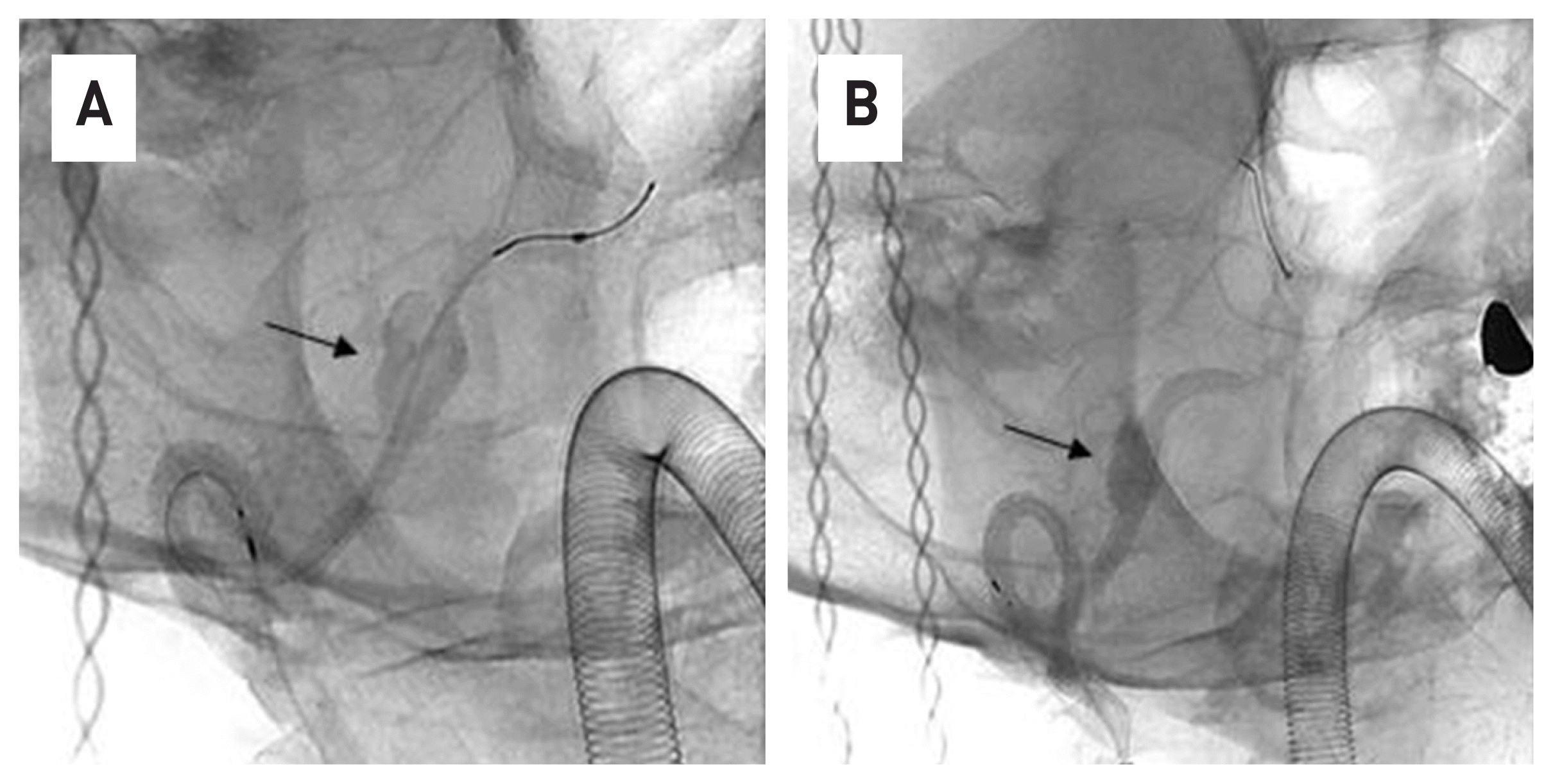
Fig. 3
Compared to Figure 1, it can be confirmed that the cerebral aneurysm was completely occluded in 3 months. (black circle)
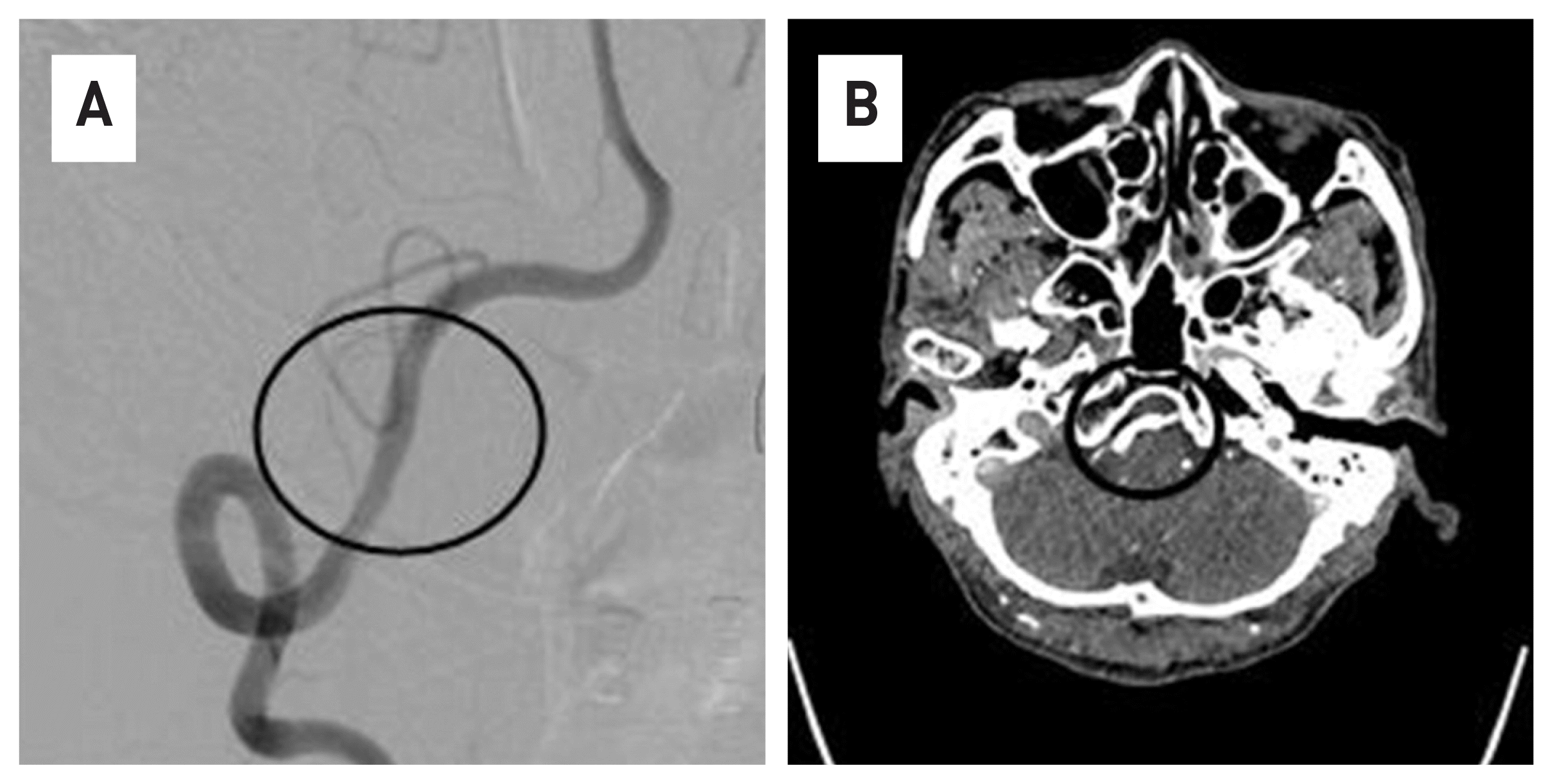
Fig. 4
VADA is observed with vessel flap in Brain MRI(A), brainstem is being pressed due to mass effect and large VADA are identified in 4-vessel angiography. (B) It is observed that FDD was deployed, including VADA lesions. (C) The end point of the stent is indicated by a white arrow.) Image (D) is an immediately after the procedure. Stagnation of contrast agents is observed as an aneurysm sac.artery.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download