Abstract
Refractory ascites is a rare complication after liver transplantation, and its incidence ranges from 5% to 7%. A 56-year-old man diagnosed with HBV-LC with massive ascites underwent living donor liver transplantation. After transplantation, more than 1000 ml/day of ascites was steadily drained until two weeks after LT. CT showed intrahepatic Rt. portal vein thrombosis and many remnant collaterals with splenomegaly. We decided to embolize the proximal splenic artery and use apixaban to reduce portal flow and resolve the intrahepatic portal thrombosis. One day after splenic artery embolization, the patient’s ascites dramatically decreased. Three days later, he was discharged from the hospital. Three months later, a follow-up liver CT showed resolution of thrombosis and no ascites. Splenic artery embolization was an effective and safe procedure for portal flow modulation in portal hyertension. Apixaban was effective for partial portal vein thrombosis in a liver transplant recipient.
Go to : 
Ascites is common and usually disappear within several days after liver transplantation (LT). However, refractory ascites (RA) is rare and ranges from 5% to 7%.1 Hepatic inflow and outflow impairments are the most common causes of RA.2 Portal hypertension, acute and chronic rejection, recurrent hepatitis, heart failure, recurrent intra-abdominal infections, renal insufficiency, electrolyte imbalances, and a failure to thrive are commonly related to refractory ascites.3 Here, we report a case of RA in a patient with intrahepatic portal thrombosis after living donor liver transplantation (LDLT).
A 56-year-old male was diagnosed with hepatitis B virus (HBV)-liver cirrhosis (LC) with ascites. He had many kinds of collateral vessels, including esophageal varix and splenomegaly, on abdominal computed tomography (CT). On 28 March 2019, we performed LDLT. His wife, a 50-year-old female, donated her Rt. lobe, which weighed 704 g with fatty change less than 5%. The Graft Recipient Weight Ratio (GRWR) was calculated as 1.05%. The venoplasty was done for a single orifice of the right hepatic vein. The branches of the middle hepatic vein (V5, V8) were anastomosed to inferior vena cava using the cryopreserved iliac vein graft. The right portal vein (PV) of the graft was anastomosed to the main PV of the recipient, and a duct-to-duct anastomosis was performed for the bile duct reconstruction. We did collateral vein ligation for inhibiting PV flow steal during the operation. Basiliximab was used as an induction immunosuppressive drug, and methylprednisolone, mycophenolic acid, and FK506 were used as maintenance immunosuppressive drugs. The target FK level was 8–10 ng/dl, and the doses of the maintenance immunosuppressive drugs were tailored to the patient’s condition. On postoperative day 10, the patient’s total bilirubin had increased from 4mg/dl up to 9 mg/dl, and a liver biopsy revealed acute cellular rejection. He received steroid pulse therapy, and his total bilirubin level normalized.
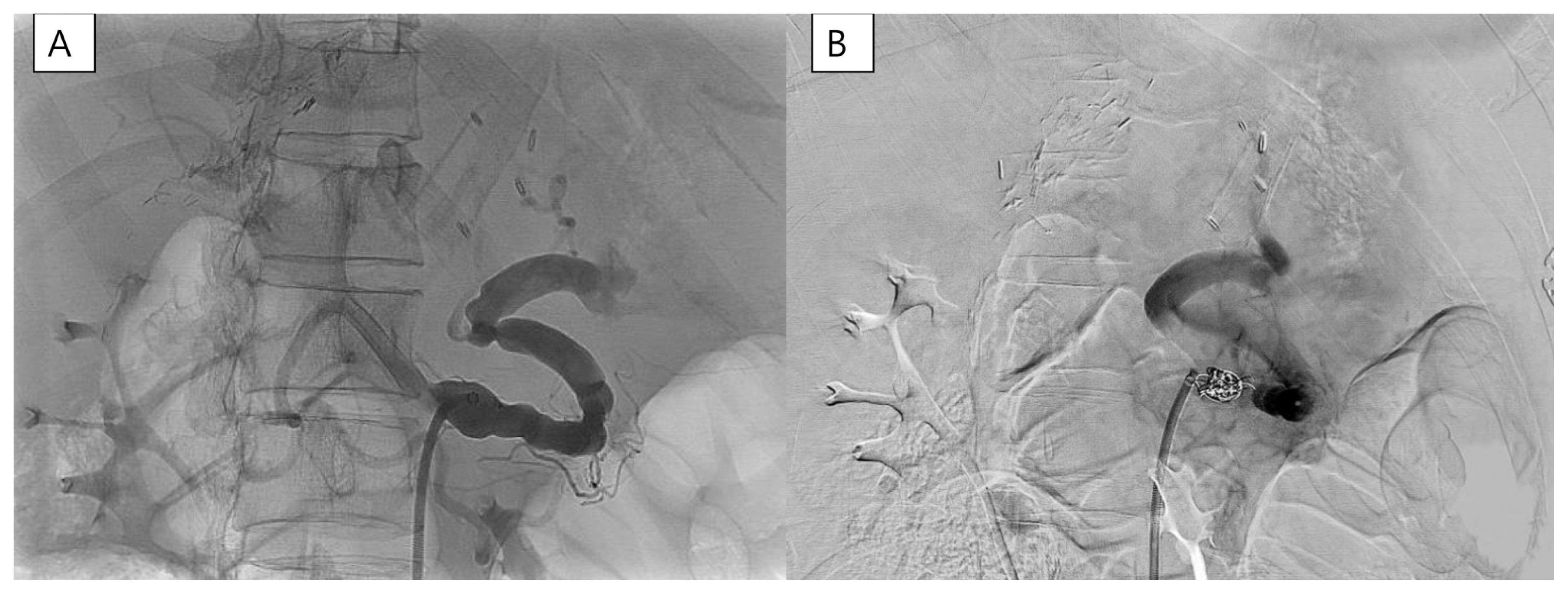
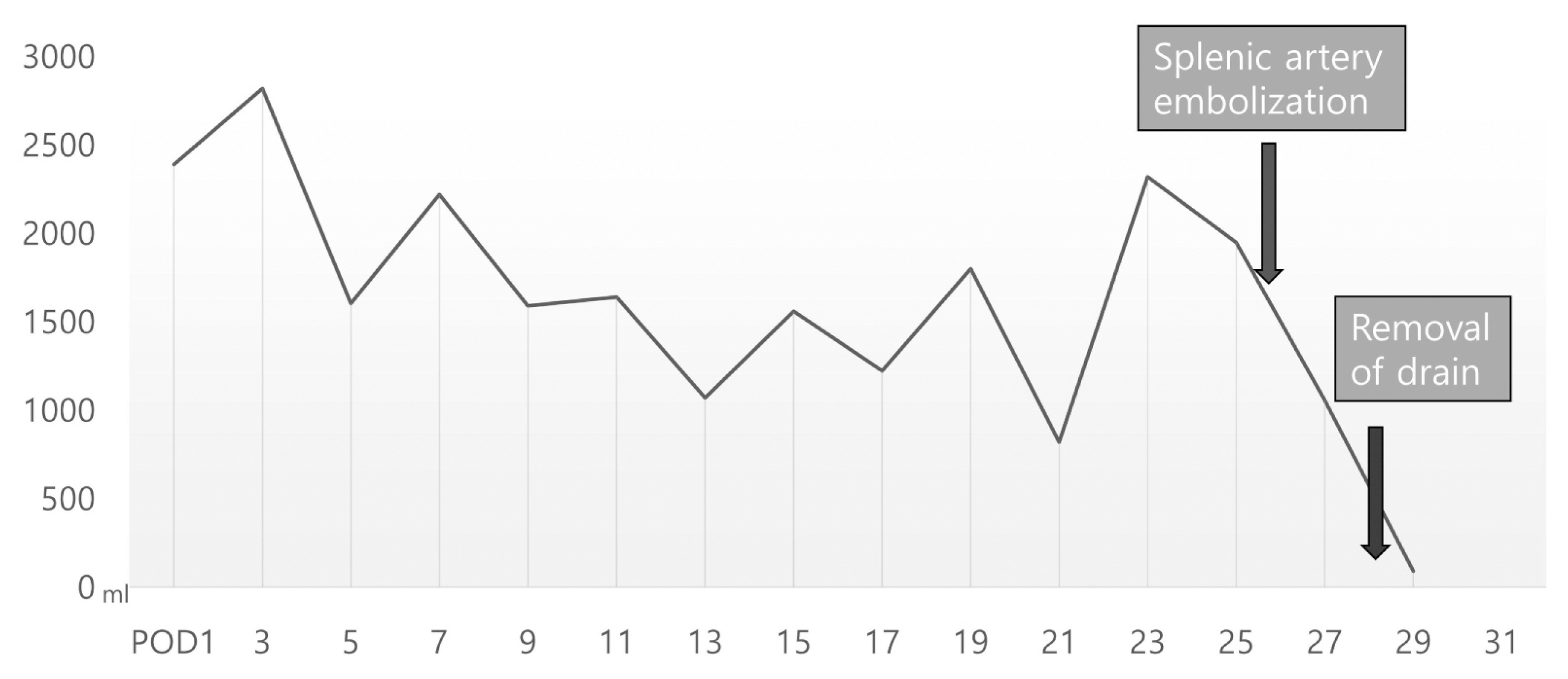
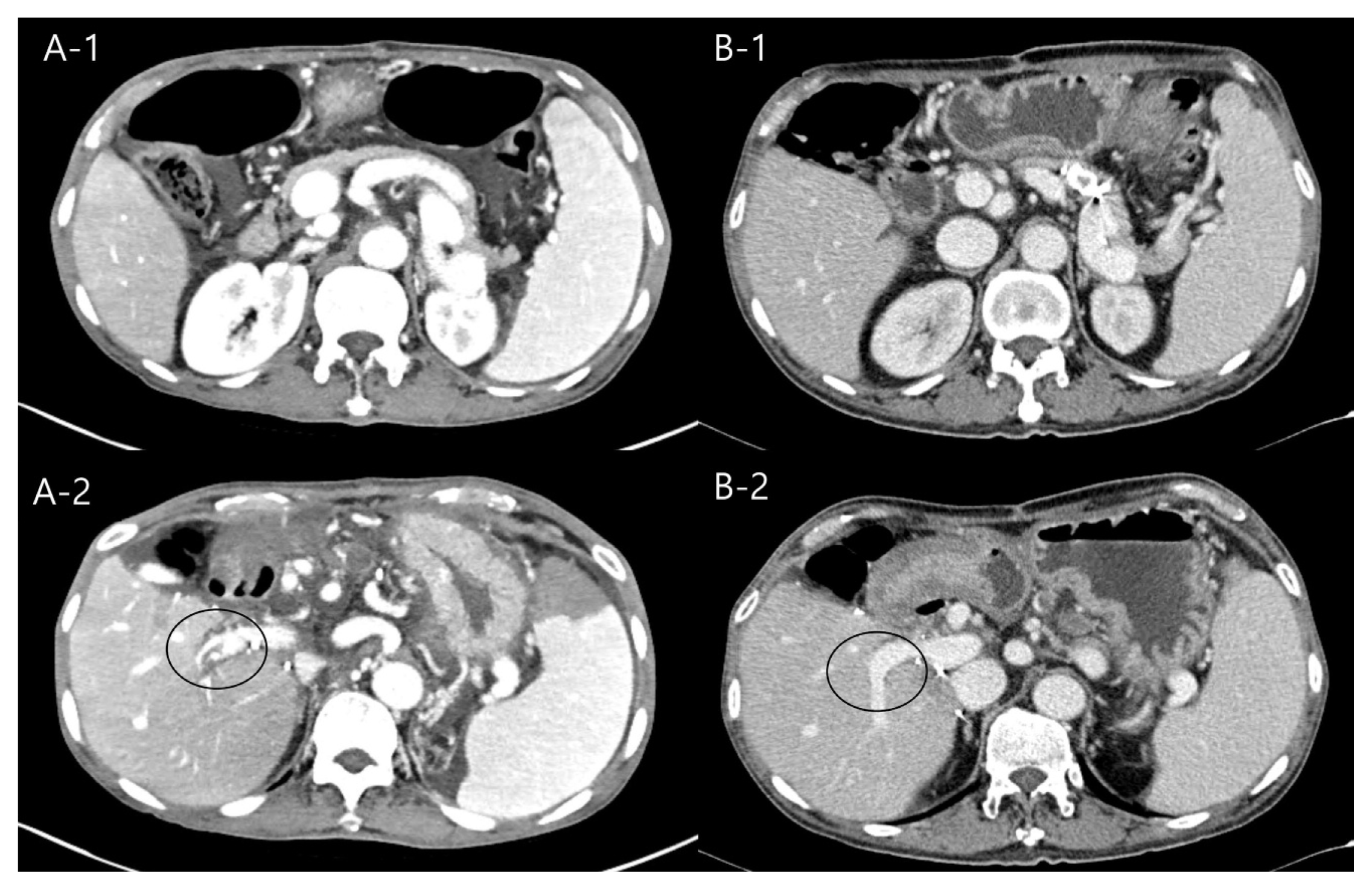
The patients had a large amount of ascites from before LT. The volume of ascites measured 5000 ml during operation. The amount of ascites after LDLT had gradually decreased from 3000 ml/day to 1500 ml/day until post-transplant two weeks. However, since then, the amount of ascites has no longer decreased. Therefore, we checked an abdominal CT and liver doppler ultrasound (US). Intrahepatic right PV thrombosis was found on CT without abnormality of main PV flow and hepatic vein outflow. The thrombosis was thought to have come from the ligated collateral vein. The main PV flow was intact as 40 cm/s of peak systolic velocity (PSV), and the hepatic artery resistive index (RI) was 0.71 on doppler US. The patient’s platelet count was around 50,000/ul, the calculated spleen volume was 990 cc, and the spleen/liver ratio was 1.49. We decided to embolize the proximal splenic artery to reduce PV flow, and we used apixaban for intrahepatic PV thrombosis. Splenic artery embolization (SAE) was performed with a type II Amplatzer vascular plug (14 mm; St. Jude Medical, St. Paul, MN, USA) and two Concerto detachable coils (10 mm and 14 mm × 30 cm; Medtronic, Dublin, Ireland) (Fig. 1). One day after splenic artery embolization, the patient’s ascites dramatically decreased (Fig. 2). After SAE, it was confirmed that the PSV of the hepatic artery was lowered from 90 cm/s to 44 cm/s on doppler US. Three days later, the patient was discharged from the hospital. Three months later, the patient’s platelet count recovered to 90,000/ul, follow-up liver CT showed no thrombosis in PV, and we discontinued apixaban (Fig. 3). Until now, 20 months after surgery, the liver function has been well maintained without ascites or vascular and biliary problems.
Go to : 
A normal liver graft restores the mechanical component of portal hypertension but does not immediately restore the systemic or splanchnic circulation to normal.3 Portal hypertension raises the hydrostatic pressure within the hepatic sinusoids and promotes fluid shifting into the peritoneal cavity. Portal hypertension also induces changes in the splanchnic circulation.4 The contribution of splenic blood to PV flow in a patient with cirrhosis can increase by up to 60%.3 Luca et al.5 demonstrated that transient occlusion of the splenic artery caused a significant and marked decrease in the PV pressure gradient.
Interestingly, after splenic artery occlusion, a direct correlation was observed between the spleen volume and the reduction of the portal pressure gradient. Therefore, SAE reduces portal hyertension by diminishing portal inflow pressures and resolving graft congestion.1
Our patient had several risk factors for RA after LT. The recipient had massive ascites before transplant. The liver graft was relatively small to the recipient (GRWR was 1.05) and the donor relatively old as 50 years old. Moreover, an unexpected intrahepatic Rt. PV thrombosis occurred after the transplant. Finally, other contributing factors could include an acute rejection episode in the immediate post-transplant period, electrolyte imbalance, and malnutrition. The pre-SAE spleen volume was calculated as 990 cc, and the pre-SAE spleen/liver ratio was 1.49.
Presser et al.6 described the pre-SAE spleen/liver ratio as a predictor of PV flow reduction after splenic artery occlusion. However, a predictable reference value was not presented. In addition, the pre-SAE sleep/liver ratio of patients who needed SAE in their study averaged 0.82, which is much lower than that of our patients. This is thought to be because their research is on deceased donor liver transplantation patients. On the other hand, the pre-transplant spleen volume (≥ 829 ml) was presented as a predictor of RA, but this is also a study of deceased donor liver transplantation.7 The risk factors and potential etiologies of PV hyperperfusion remain largely unknown. Further research is needed on the cause or predictor of PV hyperperfusion.
Humar et al.8 described the positive results of splenic artery embolization for two cases of the established small-for-size syndrome (SFSS) after LDLT. Troisi et al.9 described splenic artery ligation (SAL) to help prevent small-for-size syndrome and resolve RA in LDLT. If portal hyertension is expected with SFSS, it would be better to perform portal flow modulation by performing SAL or splenectomy during LT.
In the case of our patient, ascites persisted after LT, and we did not use routine diuretics and decided to perform SAE because we considered the leading cause of RA is portal hyertension which was needed PV flow modulation. Early SAE might be helpful because RA, which lasts more than a month after liver transplantation, causes repeated hospitalization and leads to malnutrition, impaired graft liver function, and mortality.1 Fortunately, after splenic artery embolization, the ascites dramatically decreased in our patient.
Doppler US is a non-invasive test and is helpful in liver transplant patients. It is known that hepatic artery RI and PSV increase when PV hyperperfusion is present.10 In our patients, the RI value was normal before SAE, but it was confirmed that increased hepatic artery PSV improved significantly after SAE. Most patients have some degree of pain, fever, and nausea/vomiting following SAE. Several studies reported major complication rates (15–30%) after partial SAE in the trauma setting.11,12 In contrast, proximal SAE in LT preserves collateral flow to the spleen and minimizes the likelihood of splenic infarction.3
Apixaban is one of the new oral anticoagulants (NOACs), which are used as standard care for stroke prophylaxis and the treatment of acute venous thromboembolism and pulmonary embolism. 13 Janczak et al.14 suggested that NOACs could be an alternative to low molecular weight heparin and vitamin K antagonists in patients with underlying cirrhosis and PV thrombosis. Among different NOACs, apixaban is associated with a relatively lower risk of bleeding than rivaroxaban and dabigatran.15 We used apixaban from the immediate post-liver transplant period to three months after LT. Chang et al.16 reported a case in which massive ascites occurred due to portal vein thrombosis after LT in Budd-Chiari syndrome patients. Anticoagulation was maintained for main PV thrombosis from one month after LT, and 27 months later, it developed into massive ascites. After SAE, the ascites improved. From this point of view, SAE seems to be helpful in reducing ascites even in the presence of PV thrombosis.
RA were attributed to mainly portal hyertension due to pre-transplant collateral vein and development of splenomegaly, relatively old and small liver graft, and postoperative acute cellular rejection in our patients. Intraportal thrombosis was estimated to have fallen after collateral vein ligation. In this case, it is thought that it can be successfully treated with PV flow modulation through proximal SAE and apixaban use.
Go to : 
REFERENCES
1. Meighani A, Jafri S-MR, Raoufi M, Salgia R. Splenic Artery Embolization for Treatment of Refractory Ascites After Liver Transplantation. ACG Case Reports J. 2016; 3:136–8.

2. Unger LW, Mandorfer M, Reiberger T. Portal Hypertension after Liver Transplantation — Causes and Management. Curr Hepatology Rep. 2019; 18:59–66.
3. Quintini C, D’Amico G, Brown C, Aucejo F, Hashimoto K, Kelly DM, et al. Splenic Artery Embolization for the Treatment of Refractory Ascites After Liver Transplantation. Liver Transplant. 2011; 17:668–73.

4. Lee TY, Fan HL, Wang CW, Hsieh CB, Chen TW. Somatostatin Therapy in Patients with Massive Ascites After Liver Transplantation. Ann Transplant. 2019; 24:1–8.

5. Luca A, Miraglia R, Caruso S, Milazzo M, Gidelli B, Bosch J. Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transplant. 2006; 12:1237–43.

6. Presser N, Quintini C, Tom C, Wang W, Liu Q, Diago-Uso T, et al. Safety and Efficacy of Splenic Artery Embolization for Portal Hyperperfusion in Liver Transplant Recipients: A 5-Year Experience. Liver Transplant. 2015; 21:435–41.

7. Grieser C, Denecke T, Steffen IG, Avgenaki M, Fröhling V, Mogl M, et al. Multidetector computed tomography for preoperative assessment of hepatic vasculature and prediction of splenic artery steal syndrome in patients with liver cirrhosis before transplantation. Eur Radiol. 2010; 20:108–17.

8. Humar A, Beissel J, Crotteau S, Cohen M, Lake J, Payne WD. Delayed splenic artey occlusion for treatment of established small-for-size syndrome after partial liver transplantation. Liver Transplant. 2009; 15:163–8.
9. Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, et al. Modulation of Portal Graft Inflow: A Necessity in Adult Living-Donor Liver Transplantation? Ann Surg. 2003; 237:429–36.

10. Iranpour P, Lall C, Houshyar R, Helmy M, Yang A, Choi J Il, et al. Altered Doppler flow patterns in cirrhosis patients: An overview. Ultrasonography. 2016; 35:3–12.

11. Vivek MA, Augustine A, Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg. 2014; 10:62–7.
12. Van der Cruyssen F, Manzelli A. Splenic artery embolization: Technically feasible but not necessarily advantageous. World J Emerg Surg. 2016; 11:1–14.

13. Perkins JD. Techniques to ensure adequate portal flow in the presence of splenorenal shunts. Liver Transplant. 2007; 13:767–8.
14. Janczak DT, Mimier MK, McBane RD, Kamath PS, Simmons BS, Bott-Kitslaar DM, Lenz CJ, Vargas ER, Hodge DO, Wysokinski WE. Rivaroxaban and Apixaban for Initial Treatment of Acute Venous Thromboembolism of Atypical Location. Mayo Clin Proc. 2018; 93:40–7.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download