Abstract
Objectives
Rasmussen’s aneurysm may cause life-threatening hemoptysis. We investigated the clinical characteristics and outcomes of patients with hemoptysis and Rasmussen’s aneurysm.
Methods
We retrospectively investigated patients who clinically presented with hemoptysis and were diagnosed with a Rasmussen’s aneurysm on spiral chest computed tomography (CT).
Results
Our study included 16 patients (men:women, 12:4; mean age, 65.25 ± 13.0 years). Massive hemoptysis was observed in nine patients (56%) and blood-tinged sputum in four patients (25%). Ten patients (62.5%) had a history of pulmonary tuberculosis, and three patients (18.7%) had underlying lung cancer. Chest CT revealed coexisting fungal balls in seven patients (43.7%). Bronchial artery embolization (BAE) was performed in 12 patients (75%). One patient died of uncontrolled massive hemoptysis.
Massive hemoptysis is often described as life-threatening bleeding from the respiratory tract, and it represents expectoration of more than 150 mL of blood for 24 h or bleeding at a rate of > 100 mL/h.1 Although life-threatening hemoptysis is uncommon, it shows a high mortality rate of 50% – 80% if untreated.2 The most common causes are destructive lung disease caused by bronchiectasis, pulmonary tuberculosis, bronchogenic carcinoma, fungal infection, and cystic fibrosis.3
Inflammatory pseudoaneurysm of the pulmonary arteries is a rare cause of massive hemoptysis, and Fritz Walder Rasmussen first described it in patients with tuberculosis in 1868.3 They result in focal dilation of a branch of the pulmonary artery due to erosion of the arterial wall, secondary to chronic inflammation inside a tuberculosis cavity,4 leading to rupture and life-threatening massive hemoptysis with a mortality rate ranging from 50% to 100%.5–8 An autopsy report detected that 4% of patients died of pulmonary tuberculosis.9 Although Rasmussen’s aneurysm is very rarely reported, a retrospective series, including 189 patients using multi-detector CT, described a prevalence of 6.9% in tuberculosis patients with massive hemoptysis. 10 This may be the cause of massive hemoptysis, and it is not uncommon in countries with a prevalence of tuberculosis.11,12 Extensive reports have not been reported in the literature, except for random case reports, also in Korea where tuberculosis is prevalent.13–15 Therefore, we aimed to evaluate the clinical characteristics and outcomes of patients with hemoptysis due to Rasmussen’s aneurysm in a regional tertiary hospital in Korea
Patients diagnosed with Rasmussen’s aneurysm at a regional university-affiliated hospital between September 1st, 2010 and May 31st, 2020 were retrospectively enrolled in this study. Rasmussen’s aneurysm was identified based on chest findings obtained using a 64-detector computed tomography (CT) scanner (Brilliance-64; Philips Medical Systems, Eindhoven, Netherlands). A pulmonary radiologist evaluated the presence of an aneurysm in the pulmonary artery on a CT scan. Patients were excluded if they were admitted for pregnancy or were < 18 years of age.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (GNUCH-2021-03-004). The need for informed consent was waived because of the retrospective nature of the study.
We retrospectively investigated the medical chart reviews, including patient demographics, clinical presentations, laboratory data, coexisting underlying diseases, microbiological data, and clinical outcomes. We also assessed whether patients underwent bronchial artery embolization, and recurrence of hemoptysis was assessed after the procedure.
Sixteen patients (men:women, 12:4; mean age, 65.25 ± 13.0 years) were identified. Hemoptysis was documented in all patients, and the estimated amount of hemoptysis was blood-tinged sputum in four patients (25%), < 100 cc for 24 h in 3 patients (18.7%), and > 150 cc for 24 h in nine patients (56%). Ten patients (62.5%) had a previous history of pulmonary tuberculosis(TB), and three patients (18.7%) had underlying lung cancer. Two patients were diagnosed with active pulmonary tuberculosis, and Mycobacterium tuberculosis was cultured in sputum or bronchial washing fluid. In one patient, Mycobacterium intracellulare was cultured in bronchial aspirate fluid and sputum. Two patients were administered aspirin and/or antiplatelet agents to treat coexisting cardiovascular diseases. Aspergillus antibodies were detected in the serological tests of six patients.
The location of the aneurysm was mostly in right and left upper lobes in 13 patients and the size was variable from 0.2 cm to 2 cm (mean 0.88 ± 0.57cm). A size over 1cm of the aneurysm was noted in 5 patients. These patients showed hemoptysis over 100cc/24hours. Chest CT revealed coexisting fungal balls in seven patients (43.7%). Selective angiographic embolization was performed in 12 patients (75%), of which 5 patients showed shunt communicating bronchial and pulmonary arteries on angiographic examination (Fig. 1, 2), and 11 patients exhibited improved hemoptysis after the procedure. Bronchoscopic examinations were performed in 10 patients. Bronchoscopic biopsies were performed in four patients, resulting in the discovery of lung cancer in three patients.
One patient required tracheal intubation and mechanical ventilation application because of respiratory distress associated with massive hemoptysis. One patient underwent an operation to control the cause of hemoptysis after angiographic embolization. One patient died of uncontrolled massive hemoptysis, and two patients died of respiratory failure due to the progression of the underlying lung disease (Table 1).
This study described Rasmussen’s aneurysm, in which the majority presented with massive hemoptysis in clinical practice. A substantial portion of patients was performed to control bleeding with selective angiographic embolization using a coil, which can be controlled with this intervention and conservative management.
In our study, 12 patients were active or had a previous history of pulmonary TB. In patients with a history of pulmonary tuberculosis, hemoptysis could be responsible for bronchiectasis, Aspergillus infection in the cavity, and reactivation of tuberculosis. In these patients, massive or life-threatening hemoptysis can occur with complicated vascular problems and it usually originates from the bronchial circulation rather than the pulmonary circulation. In contrast, Rasmussen’s aneurysm is a pseudoaneurysm of the pulmonary artery caused by gradual weakening and thinning of the pulmonary arterial wall, resulting in pseudoaneurysm formation. In addition, bronchial arteries due to chronic inflammation may lead to vessel erosion and develop arteriovenous or bronchopulmonary shunts, which communicate with the bronchial and pulmonary arteries. It may cause pulmonary artery pseudoaneurysm. 16,17 In our study, five patients showed a shunt communicating with the bronchial and pulmonary arteries. In such cases, selective embolization of the bronchial artery may result in effective control of bleeding in patients with massive hemoptysis due to Rasmussen’s aneurysm. In cases of Rasmussen’s aneurysm, non-communicating bronchial or systemic artery, direct embolization of the aneurysm via pulmonary artery may be effective in controlling bleeding.18 In a peripheral aneurysm, percutaneous transthoracic embolization has been reported to be an effective alternative method to endovascular embolization. 19
Rasmussen’s aneurysm is a vascular complication of pulmonary tuberculosis with cavity formation and it is associated with previous and recent active pulmonary tuberculosis.20 A review of autopsy findings showed a 5% prevalence of Rasmussen aneurysm in patients with a history of tuberculosis with a chronic cavitary lesion.21
The long-term effects of angiographic embolization have not been extensively demonstrated in the literature. In our study, only one patient underwent surgery to control bleeding. Most patients showed immediate improvement with embolization and conservative management. Therefore, even though life-threatening hemoptysis can develop because of Rasmussen’s aneurysm, angiographic embolization can immediately control the bleeding from this pathological disease.
In this study, coexisting fungus balls were noted in seven patients via CT scans, and the Aspergillus antibody test was positive in five patients. Serum Aspergillus antibodies can be detected in chronic pulmonary aspergillosis infections, such as aspergilloma and invasive and semi-invasive aspergillosis. It is usually associated with old tuberculosis infection of the lung, which causes a destructive change in the lung parenchyma with cavity formation. It can be a nidus in the development of Aspergillus infection and the formation of pseudoaneurysm.22,23
Rasmussen’s aneurysm can arise from lung cancer as a consequence of vascular invasion, destruction, and tissue necrosis.24,25 In our study, lung cancer was diagnosed in 3 patients. In some cases, it may be associated with chemotherapy, radiotherapy, or radiofrequency ablation.26,27 In our study, patients were diagnosed with lung cancer for the first time in the hospital. Therefore, the development of an aneurysm may be associated with combined lung cancer that is unrelated to the previous treatment.
Surgical resection may be an alternative treatment strategy for uncontrolled massive hemoptysis and prevention of recurrent bleeding.2,28 In patients with Rasmussen’s aneurysm, surgical resection can be performed in selected cases, such as those with preserved lung function accompanied by recurrent and uncontrolled life-threatening hemoptysis, as stated by only a few reports in the literature.4,6,22 Despite aggressive surgical treatment, the clinical outcome is very poor in most situations. In our study, one patient underwent surgical resection after successful embolization of the aneurysm.
In conclusion, Rasmussen’s aneurysm may be the cause of massive hemoptysis. Angiographic embolization in selected cases could be an effective therapeutic strategy to control bleeding in most patients. Long-term clinical outcomes should be clarified in the future.
REFERENCES
1. Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J. 2008; 32:1131–2.

2. Amirana M, Frater R, Tirschwell P, Janis M, Bloomberg A, State D. An aggressive surgical approach to significant hemoptysis in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1968; 97:187–92.
3. Rasmussen V, Moore WD. On Hæmoptysis, Especially When Fatal, in Its Anatomical and Clinical Aspects. Edinb Med J. 1868; 14:486–503.
4. Shih SY, Tsai IC, Chang YT, Tsan YT, Hu SY. Fatal haemoptysis caused by a ruptured Rasmussen’s aneurysm. Thorax. 2011; 66:553–4.

5. Syed M, Irby J. Airway management of ruptured pulmonary artery “Rasmussen” aneurysm and massive hemoptysis. BMC Res Notes. 2015; 8:346.

6. Patankar T, Prasad S, Deshmukh H, Mukherji SK. Fatal hemoptysis caused by ruptured giant Rasmussen’s aneurysm. AJR Am J Roentgenol. 2000; 174:262–3.

7. Basille D, Andrejak C, Gosset M, Renard C, Jounieaux V. Severe haemoptysis revealing a Rasmussen aneurysm. Rev Mal Respir. 2010; 27:63–6.
8. Neelakantan S, Anandarajan R, Swamy AK. Rare cause of massive haemoptysis in pulmonary tuberculosis: Rasmussen’s aneurysm. BMJ Case Rep. 2016; 2016.

9. Auerbach O. Pathology and pathogenesis of pulmonary arterial aneurysm in tuberculous cavities. Am Rev Tuberc. 1939; 39:99–115.
10. Corr P. Pulmonary artery aneurysm as a cause of massive hemoptysis: diagnosis and management. Case reports in radiology 2011. 2011.
11. Peghini Gavilanes E, Lopez Yepes LA, Penalver Paolini CL, Morales Ruiz R. Rasmussen’s pseudoaneurysm in a patient with a history of pulmonary tuberculosis. Arch Bronconeumol. 2015; 51:96–7.

12. Giraldo-Montoya ÁM, Rodríquez-Morales AJ, Hernández-Hurtado JD, López-Salazar Á, Lagos-Grisales GJ, Ruiz-Granada VH. Rasmussen aneurysm: A rare but not gone complication of tuberculosis. Int J Infect Dis. 2018; 69:8–10.

13. Lee JR, Lee SH, Jung SH, Song SH, Kim CH, Moon HS, et al. A Case of Endobronchial Mass-Like Rasmussen Aneurysm. Tuberculosis and Respiratory Diseases. 2004; 56:85–90.

14. Park SO, Ko H, Kim SH, Park W, Lee DH, Ryu DS, et al. A Case of Rasmussen Aneurysm Treated by Pulmonary Arterial Embolization. Tuberculosis and Respiratory Diseases. 2001; 51:53–8.

15. Shin K-C, Chung J-H. case of massive hemoptysis due to Rasmussen aneurysm and successful embolization with micro-coil. The Korean Journal of Medicine. 2008; 74:110–1.
16. Davidoff AB, Udoff EJ, Schonfeld SA. Intraaneurysmal embolization of a pulmonary artery aneurysm for control of hemoptysis. AJR Am J Roentgenol. 1984; 142:1019–20.

17. Lundell C, Finck E. Arteriovenous fistulas originating from Rasmussen aneurysms. AJR Am J Roentgenol. 1983; 140:687–8.

18. Tanahashi Y, Kondo H, Osawa M, Yamamoto T, Yamaguchi M, Furui S. Transcatheter embolization of a Rasmussen aneurysm via pulmonary artery with n-butyl cyanoacrylate and iodized oil mixture injection with balloon occlusion. J Vasc Surg Cases Innov Tech. 2016; 2:161–4.

19. Lal A, Bansal A, Chaluvashetty SB, Sandhu MS, Gorsi U. Percutaneous transthoracic embolisation for massive haemoptysis secondary to peripheral pulmonary artery pseudoaneurysms. Eur Radiol. 2021; 31:2183–90.

20. Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001; 21:839–58. discussion 59–60.

21. Santelli ED, Katz DS, Goldschmidt AM, Thomas HA. Embolization of multiple Rasmussen aneurysms as a treatment of hemoptysis. Radiology. 1994; 193:396–8.

22. Wang W, Gao L, Wang X. Rasmussen’s aneurysm with aspergilloma in old, healed pulmonary tuberculosis. Clin Imaging. 2013; 37:580–2.

23. Caulet S, Capron F, Laaban JP, Prudent J, Rochemaure J, Diebold J. Fatal hemoptysis during bronchial aspergillosis with multiple pulmonary artery aneurysms. Ann Pathol. 1990; 10:177–80.
24. Akpinar E, Turkbey B, Canyigit M, Peynircioglu B, Hazirolan T, Pamuk AG, et al. Bleeding pulmonary artery pseudoaneurysm secondary to squamous cell lung cancer: computed tomography findings and endovascular management. Acta Radiol. 2006; 47:944–6.

25. Padrones SS, Lisbona RL, Gratacos AR, Rodriguez AN, Diaz-Jimenez JP. Pulmonary artery pseudoaneurysm arising from a lung tumor. J Bronchology Interv Pulmonol. 2009; 16:274–6.

26. Kim JH, Han SH. A pulmonary artery pseudoaneurysm caused by concurrent chemoradiation therapy for lung cancer. Pak J Med Sci. 2015; 31:220–2.

Fig. 1
A 59-year-old man presented with massive hemoptysis amounting to approximately 100 cc. (A) Chest X-ray showing volume loss in the right lung with a cavitary lesion in the upper lobe. A calcified lesion in the field of the left upper lobe represents the previous history of pulmonary tuberculosis. (B) Enhanced chest CT scan showing cavitary lesion with sponge-like soft tissue in the collapsed right upper lobe. A focal aneurysm in the area adjacent to the cavitary lesion represents Rasmussen’s aneurysm.
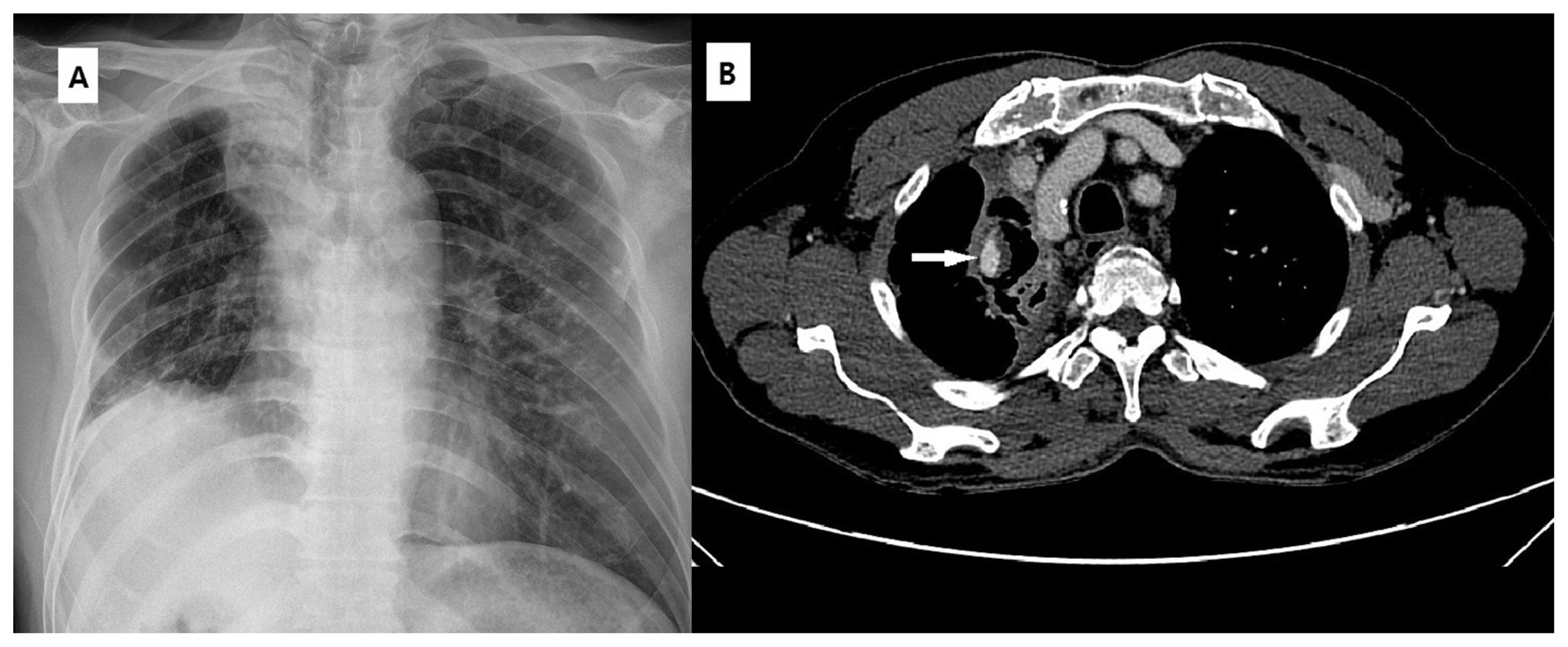
Fig. 2
An 82-year-old man with lung cancer complained of hemoptysis for 3 days. (A) Chest X-ray showing dense a consolidative lesion in the left upper lobe with an air-space lesion. (B) Enhanced chest CT scan showing diffuse consolidation and necrotic change in the left upper lobe and formation of Rasmussen’s aneurysm in the necrotic and consolidative lesion. (C) The angiographic finding was a large aneurysm in the left pulmonary artery branch.
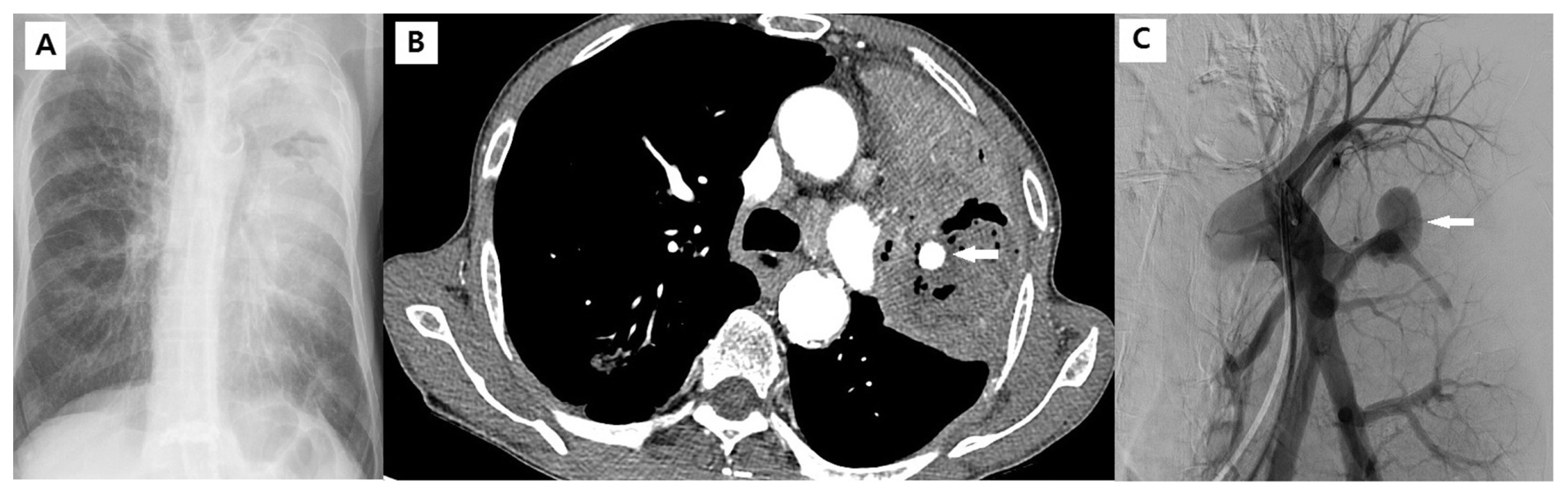
Table 1
The clinical characteristics and outcome in 16 patients with Rasmussen’s aneurysm
BAE, bronchial artery embolization; CVA, cerebrovascular accident; DM, diabetes mellitus; CKD, chronic kidney disease; MV, Mechanical ventilation; TB, tuberculosis; CHF, congestive heart failure; LC, liver cirrhosis; COPD, chronic obstructive pulmonary disease; S, survived; D, died; RUL, right upper lobe; LUL, left upper lobe; RLL, right lower lobe; LLL, left lower lobe




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download