Abstract
Partially thrombosed intracranial aneurysm was difficult to treat because of higher recurrence rate compared to non-thrombosed saccular aneurysm. The author reports a case of partially thrombosed intracranial aneurysm causing transient ischemic symptom. A 40-year-old man presented with transient right hemiparesis. Brain magnetic resonance imaging (MRI) depicted low-signal intensity target-like mass lesion on left sylvian fissure, and magnetic resonance angiography (MRA) showed aneurysm on left middle cerebral artery bifurcation (MCBF), suggested thrombosed aneurysm. On operative finding, aneurysm wall had thick and atherosclerotic change, and it was fusiform aneurysm not saccular type. We initially planned direct clip for the aneurysm, but it was failed due to collapse of parent artery after clipping on aneurysm neck. To prevent ischemia, extracranial-intracranial bypass was performed and then thrombectomy with clip reconstruction. To remodeling the fusiform aneurysm, stent-assisted coiling was performed for remnant portion of aneurysm. With staged hybrid technique, giant thrombosed fusiform aneurysm was completely obliterated and the patient did not suffer any neurologic symptoms no longer.
Partially thrombosed intracranial aneurysms (PTIA) are characterized by intraluminal thrombosis with a solid mass [3]. These aneurysms are usually large to giant and cause neurologic deficit related with mass effect [3]. It is difficult to treat compared to classic saccular aneurysm so that two thirds of thrombosed aneurysm require additional surgical procedures such as thrombectomy with clip reconstruction or bypass-occlusion [15]. Thrombectomy with clip reconstruction is defined as trapping aneurysm with temporary clip, thrombectomy, and reconstruction of aneurysm neck with permanent clip. Thrombectomy has ischemia risk due to temporary clipping, and back bleeding can occur if there is insufficient temporary clipping, and once the aneurysm wall is opened, the decision cannot be reversed [12]. Direct clipping could occur the post-operative neurological deficit from occlusion of perforating arteries or decreasing the flow of parent artery [12]. Temporary clipping of parent artery for thrombectomy takes more time, which can increase ischemia risk [12]. Insurance bypass could be an additional option for safety removal of thrombosed giant aneurysm. We introduce a case managed with staged hybrid treatment for symptomatic giant fusiform aneurysm which performed the thrombectomy with clip reconstruction after insurance bypass and then stent-assisted coiling later.
A 40-year-old-man presented with transient right hemiparesis and motor aphasia for 5 minutes. Neurological examination showed no obvious neurological deficit. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of brain were performed for evaluation of intracranial lesion. MRA showed aneurysm on left middle cerebral artery bifurcation (MCBF) (Fig. 1A). A T2-weighted axial image showed an 18×13 mm sized low signal intensity target-like mass lesion (Fig. 1B). Gadolinium-enhanced brain vessel wall MRI showed enhancement of inner and outer wall layer of aneurysm, suggesting thrombosed aneurysm (Fig. 1C). Diagnostic cerebral angiography showed canalized portion sized 6.8×7.5 mm (Fig. 2).
The author considered a direct clipping for giant thrombosed aneurysm. Under general anesthesia with intravenous continuous injection of Propofol and Remifentail, motor evoked potential (MEP) and sensory evoked potential (SEP) was monitored during operation. For insurance bypass, frontal and parietal branch of left superficial temporal artery (STA) were dissected. Left frontotemporal craniotomy, opening dura mater, and lateral to medial sylvian dissection were performed. Aneurysm, M1 segment of middle cerebral artery (MCA), frontal and temporal branch of M2 segment of MCA were exposed. The aneurysm wall was thick with atherosclerosis, and aneurysm size was about 23 mm, larger than that measured in MRI. In addition, the aneurysm was not saccular type, but rather giant fusiform aneurysm with neck involving right M1 and frontal branch of right M2 (Fig. 3A).
Direct clip was performed first, but parent artery was collapsed together. The author thought that direct clip for this thrombosed aneurysm was impossible, and the time of temporary clipping for thrombectomy was also expected to be long. Thus, insurance bypass between parietal branch of STA and temporal branch of proximal M2 was performed to prevent ischemia of infarction during thrombectomy (Fig. 3B). After temporary clipping on M1 and both M2, aneurysm wall was opened and thrombectomy was performed (Fig. 3C). After thrombectomy, the aneurysm wall was redundant to apply permanent clip. However, MEP on right upper and lower extremities was drop down when permanent clipping of aneurysm was performed too close to the parent artery. Since the aneurysm was atherosclerotic fusiform aneurysm with thick wall, we planned clipping the aneurysm neck not tightly, and then endovascular remodeling later. So, permanent clip was repositioned to little further from the aneurysm neck (Fig. 3D), and MEP was recovered to baseline (Fig. 3E). Intraoperative cerebral angiography showed aneurysm sac was not filled with contrast except for aneurysm neck, and cerebral blood flow of left MCA territory was intact. There was no neurologic deficit after operation.
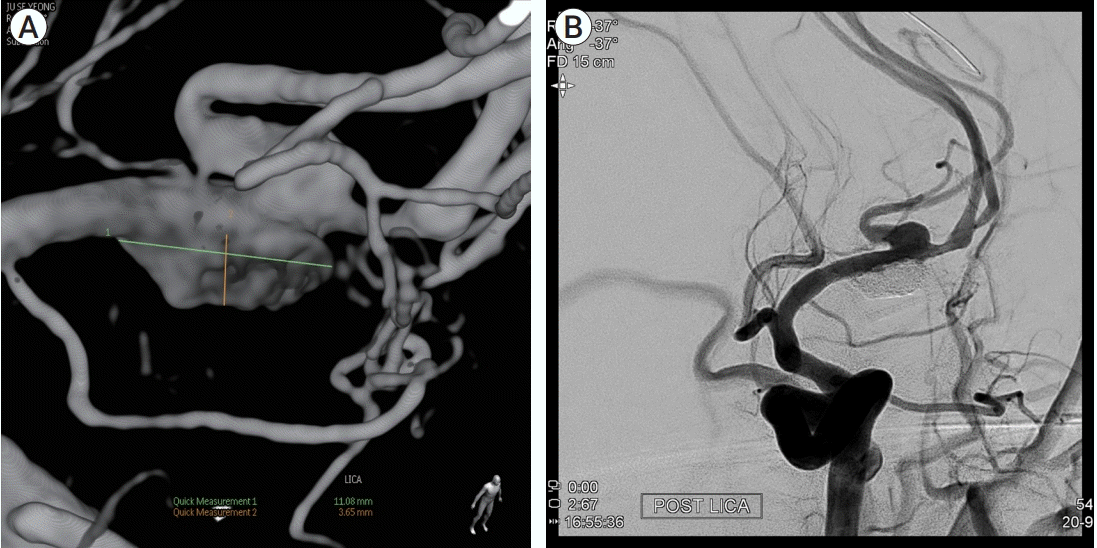
Three months after operation, he presented 2 times of transient right hemiparesis. Follow-up angiography showed re-growth of the aneurysm (Fig. 4A), so we performed stent-assisted coiling. Aneurysm sac was selected with Two Excelsior SL-10 microcatheter (Target Therapeutic, Boston Scientific, Fremont, CA, USA) was introduced to aneurysm sac. Deployment of 4.0×24 mm sized Atlas (Stryker, Fremont, CA, USA) stent was performed from temporal branch of M2 to distal M1 segment to cover aneurysm neck. Then, continuous coil packing with Hypersoft 3D and Hypersoft Helix (MicroVention, Aliso Viejo, CA, USA) was performed until the aneurysm sac was completely obliterated (Fig. 4B). The patient was neurologically intact and no longer present ischemic symptom after stent-assisted coiling. The aneurysm was completely obliterated on follow-up angiography on 6-months after coiling, and the patient no longer had suffered TIA symptoms with modified Rankin scale 0.
Treatment of PTIA is challenging compared to classic saccular aneurysm [3,17]. Endovascular treatment of PTIA has related high recurrence and retreatment rate [3,5]. In accordance a study, recurrence rate of coiled PTIA was 75% and the retreatment rate was 63% [3], whereas recurrence rate of coiled classic saccular aneurysm was 21%, and retreatment rate was 10% [4]. It was likely caused by migration of coil mass into intraluminal thrombus [7], and high regrowth rate of thrombosed aneurysm. Occlusion of parent artery is more effective to reduce recurrence rate and mass effect of thrombosed aneurysm, but it is not always possible in case that parent artery supplies eloquent area. Surgical treatment was also challenging when PTIA was located in posterior circulation, has a wide neck to inappropriate clipping, calcification, or aneurysm dome imbedded in deep parenchyma [9].
Pathogenesis of thrombosed aneurysm was not clearly demonstrated, but some authors had suggested the following hypothesis [3,6,10,11,13,18]. First, proliferation of vasa vasorum of aneurysm wall contribute the formation of giant aneurysm and intramural hematoma [10,11]. Repeated subadventitial hemorrhage from vasa vasorum causes intramural hematoma [10,11]. Also, Iihara et al. noticed developed vasa vasorum in neck of giant PTIA and parent artery, and persistent bleeding from vasa vasorum during temporary clipping of parent artery [6]. In this hypothesis, blocking the flow of vasa vasorum by clipping aneurysm wall is necessary to prevent the growth of thrombosed aneurysm. However, this hypothesis does not explain why the size of the aneurysm decreases when the parent artery occlusion with endovascular treatment, even though it does not strictly block the vasa vasorum. The other hypothesis was dissection of the aneurysm wall caused by parent artery blood flow [3]. Histopathology of thrombosed aneurysm performed by Yasui et al. showed fresh hemorrhage between old thrombus and aneurysm wall with cleft, suggesting dissection of aneurysm wall [13,18]. However, it was also insufficient to explain when thrombosed intramural hematoma occurs with lumen of parent artery [8]. Eventually, complex mechanisms of parent artery, vasa vasorum and several other factors makes thrombosed aneurysm growing, and treatment of PTIA difficult.
Lawton et al. classified thrombosed aneurysm to make the most appropriate management strategies [12]. Thrombosed aneurysm was classified into six types on the basis of aneurysm, thrombus, and lumen morphology; type 1, saccular aneurysm with concentric thrombus; type 2, saccular aneurysm with eccentric thrombus; type 3, saccular aneurysm with lobulated thrombus; type 4, completely thrombosed aneurysm; type 5, fusiform or dolichoectatic aneurysm with canalized thrombus, and type 6, iatrogenically developed thrombosed aneurysm caused by foreign material, referred as coiled aneurysm [12]. Treatment of fusiform thrombosed aneurysm was difficult due to large neck size and de-endothelialization of the lumen of parent artery [14]. They also suggested that conventional clipping of aneurysm neck was the best treatment option, but if it was not possible, bypass with parent artery occlusion were superior to thrombectomy with clip reconstruction [12]. This type of thrombosed aneurysm is inappropriate to direct clip because it has noncompliant solid neck, and it usually need another treatment strategies such as thrombectomy with clip reconstruction or bypass-occlusion [12,14]. In our case, aneurysm was included in type 5. For this giant fusiform aneurysm, the author removed thrombus, remodeling the aneurysm with clips, and performed delayed endovascular treatment, so the patient was stable without any symptoms and aneurysm was no more recurred.
We had considered several strategies to treat the thrombosed aneurysm for this patient. First, intraluminal coil embolization was considered, but it had high recurrence and retreatment rate, and it could not reduce mass effect [3]. Second, flow-diversion had high thromboembolic risk, distal ipsilateral hemorrhage or delayed rupture risk due to incomplete neointimal growth of aneurysm wall [1,2,16]. Third, conventional aneurysm neck clipping was not amenable because it caused collapse of parent artery supplying dominant hemisphere. Finally, parent artery occlusion with high-flow bypass was also inappropriate as lateral lenticulostriate artery was originated in the distal M1, which was very close to aneurysm neck. So, we planned STA-MCA insurance bypass and thrombectomy with clip reconstruction. To reduce the ischemic risk of temporary clipping, STA-MCA bypass was performed first. When clipping tightly to the aneurysm neck, MEP decreased, so we adjust clip distal to aneurysm neck. Three months after clip and bypass surgery, stent-assisted coiling for regrowing aneurysm was performed safely due to decreased size and no intraluminal thrombus. Although long-term follow-up is needed, thrombosed aneurysm could be safely and completely treated with hybrid therapy.
The combination of a variable surgical approaches with endovascular treatment made thrombosed aneurysm treated safely and effectively, and it led to good clinical outcome of the patient. For treatment of complex aneurysm, especially PTIA, it is important to make appropriate treatment strategies with good understanding of the advantages and possible complications of each surgical and endovascular approach.
ACKNOWLEDGMENTS
This research was supported by Soonchunhyang University Fund. This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Korean government (NRF-2019M3E5D1A02069061, NRF-2020R1F1A1066362) and by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea (202015X17)).
REFERENCES
1. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G, et al. Spontaneous delayed migration/shortening of the pipeline embolization device: report of 5 cases. American Journal of Neuroradiology. 2013; Dec. 34(12):2326–30.

2. Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg. 2012; Jul. 117(1):37–44.

3. Ferns SP, van Rooij WJ, Sluzewski M, van den Berg R, Majoie CBLM. Partially thrombosed intracranial aneurysms presenting with mass effect: long-term clinical and imaging follow-up after endovascular treatment. American Journal of Neuroradiology. 2010; Aug. 31(7):1197–205.

4. Ferns SP, Sprengers MES, van Rooij WJ, Rinkel GJE, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009; Aug. 40(8):e523–9.
5. Güresir E, Wispel C, Borger V, Hadjiathanasiou A, Vatter H, Schuss P. Treatment of partially thrombosed intracranial aneurysms: single-center series and systematic review. World Neurosurgery. 2018; Oct. 118:e834–41.

6. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum: case report. J Neurosurg. 2003; Feb. 98(2):407–13.
7. Iihara K, Murao K, Yamada N, Takahashi JC, Nakajima N, Satow T, et al. Growth potential and response to multimodality treatment of partially thrombosed large or giant aneurysms in the posterior circulation. Neurosurgery. 2008; Nov. 63(5):832–44.

8. Kim HJ, Lee SW, Lee TH, Kim YS. Huge intramural hematoma in a thrombosed middle cerebral artery aneurysm: a case report. Journal of Cerebrovascular and Endovascular Neurosurgery. 2015; 17(3):234–8.

9. Kim YJ, Ko JH. Endovascular treatment of a large partially thrombosed basilar tip aneurysm. Journal of Korean Neurosurgical Society. 2012; Jan. 51(1):62–5.

10. Krings T, Lasjaunias P, Geibprasert S, Pereira V, Hans FJ. The aneurysmal wall. The key to a subclassification of intracranial arterial aneurysm vasculopathies? Interventional Neuroradiology. 2008; Sep. 14(1_suppl):39–47.
11. Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neurosurgery. 2005; Dec. 47(12):931–7.

12. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery. 2005; Mar. 56(3):441–54.

13. Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg. 1995; May. 82(5):796–801.

14. Scerrati A, Sabatino G, Della Pepa GM, Albanese A, Marchese E, Puca A, et al. Treatment and outcome of thrombosed aneurysms of the middle cerebral artery: institutional experience and a systematic review. Neurosurgical Review. 2019; Sep. 42(3):649–61.

15. Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery. 2011; Dec. 69(6):1261–70.

16. Szikora I, Turányi E, Marosfoi M. Evolution of flow-diverter endothelialization and thrombus organization in giant fusiform aneurysms after flow diversion: a histopathologic study. American Journal of Neuroradiology. 2015; Sep. 36(9):1716–20.

17. Yang K, Park JC, Ahn JS, Kwon DH, Kwun BD, Kim CJ. Characteristics and outcomes of varied treatment modalities for partially thrombosed intracranial aneurysms: a review of 35 cases. Acta Neurochirurgica. 2014; Sep. 156(9):1669–75.

18. Yasui T, Sakamoto H, Kishi H, Komiyama M, Iwai Y, Yamanaka K, et al. Rupture mechanism of a thrombosed slow-growing giant aneurysm of the vertebral artery - case report. Neurologia Medico-Chirurgica. 1998; Dec. 38(12):860–4.
Fig. 1.
(A) Initial magnetic resonance angiography (MRA) showed fusiform aneurysm of left middle cerebral artery bifurcation (MCBF). (B) T2-weighted axial image showed a 22×13 mm sized low signal intensity target-like lesion on left MCBF. (C) Gadolinium-enhanced brain vessel wall magnetic resonance imaging (MRI) showed enhancement of inner and outer layer of aneurysm.
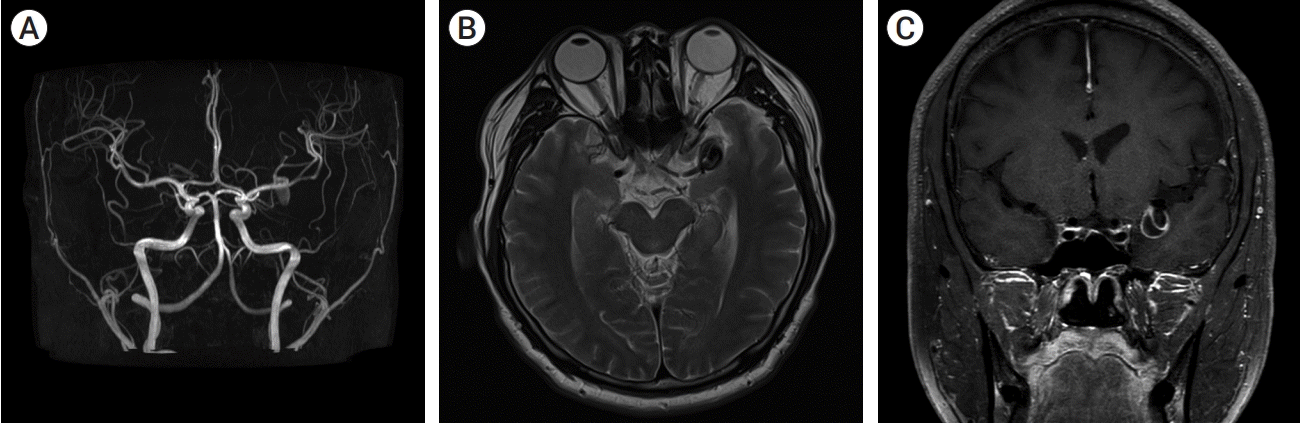
Fig. 2.
Digital subtraction angiography was performed and a 9.7×14.5 mm sized fusiform aneurysm was found on left middle cerebral artery bifurcation.
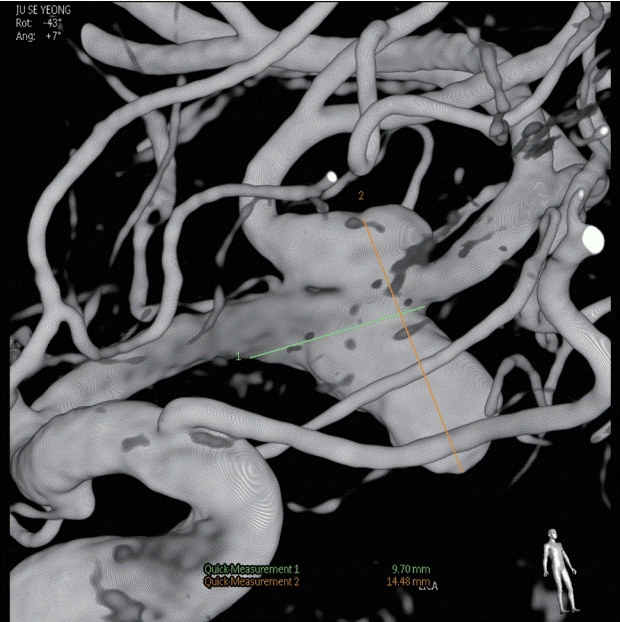
Fig. 3.
After left frontotemporal craniotomy and sylvian dissection, left M1 segment of MCA, frontal and temporal branch of M2 segment, and giant aneurysm of left MCBF were exposed. (A) Schematic imaging of fusiform aneurysm of left MCBF. Direct clip was initially performed, but left M1 was also collapsed. (B) To prevent ischemia caused by long time of temporary clipping, anastomosis of parietal branch of STA and temporal branch of M2 was performed. (C) After temporary clipping of parent artery, thrombectomy was performed. Clip reconstruction with multiple permanent clip was performed, but proximal clip on aneurysm neck block the blood flow of parent artery. (D) Reposition of clip proximal to aneurysm neck was performed. (E) MEP of right extremities was recovered to baseline after clip reposition. MCA, middle cerebral artery; MCBF, middle cerebral artery bifurcation; STA, superficial temporal artery; MEP, motor evoked potential.
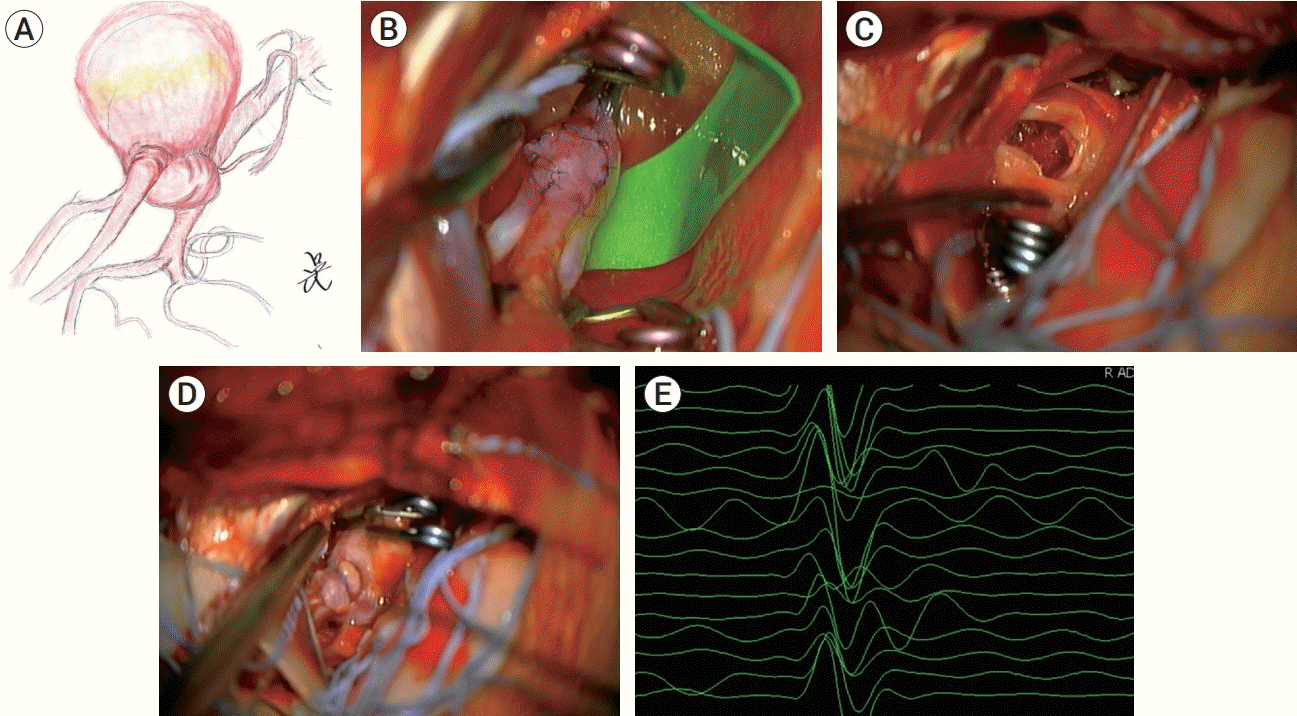
Fig. 4.
Three months after aneurysm neck clip, follow up digital subtraction angiography was performed. (A) Aneurysm was slightly regrown, so stent-assisted coiling was performed. (B) Aneurysm was completely obliterated except for the neck of the aneurysm branching frontal branch of M2 segment of left middle cerebral artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download