This article has been
cited by other articles in ScienceCentral.
Abstract
A Barrow type D of complex cavernous sinus dural arteriovenous fistula (CS-dAVF) was completely obliterated by using coils, n-butyl 2-cyanoacrylate (NBCA) and Onyx via transvenous approach. Especially in this case, after transvenous coil embolization of the pathologic cavernous sinus (CS), transvenous injection of NBCA was done to obliterate residual shunts recruited into CS. The complex CS-dAVF was completely obliterated without periprocedural complications. Transvenous injection of NBCA could be considered as a feasible option for obliteration of pathologic CS in a case of incompletely obliterated complex CS-dAVF after transvenous coil embolization.
Go to :

Keywords: Cavernous sinus, Dural arteriovenous fistula, n-butyl 2-cyanoacrylate, Onyx
INTRODUCTION
Transvenous coil embolization has been considered as a standard strategy for cavernous sinus dural arteriovenous fistula (CS-dAVF) treatment [
3]. Considering liquid embolic materials to treat dAVF, transarterial and/or transvenous injection of Onyx has recently been used to obliterate pathologic cavernous sinus in cases of dAVF, and it showed good angiographic and clinical outcomes [
1,
4,
5,
7]. We present a case of transvenous injection of n-butyl 2-cyanoacrylate (NBCA) to obliterate the pathologic cavernous sinus as a salvage technique for incompletely obliterated complex CS-dAVF after transvenous coil embolization.
Go to :

DESCRIPTION OF A CASE
A 60-year-old female patient with exophthalmos, chemosis of the right eye and diplopia secondary to right third nerve palsy visited an ophthalmologic clinic. Contrast-enhanced orbital computed tomography (CT) showed a dilated superior ophthalmic vein (SOV) in the right orbit. Brain magnetic resonance angiography (MRA) revealed prominent heterogenous vascularity at the right cavernous sinus. She was referred to our department for evaluation and treatment of the CS-dAVF. Diagnostic cerebral angiogram demonstrated a Barrow type D of complex CS-dAVF at the right side of cavernous sinus (CS) fed by meningeal branches originated from both internal carotid artery (ICA) and external carotid artery (ECA), and draining into the right SOV and the right inferior petrosal sinus (IPS) (
Fig. 1).
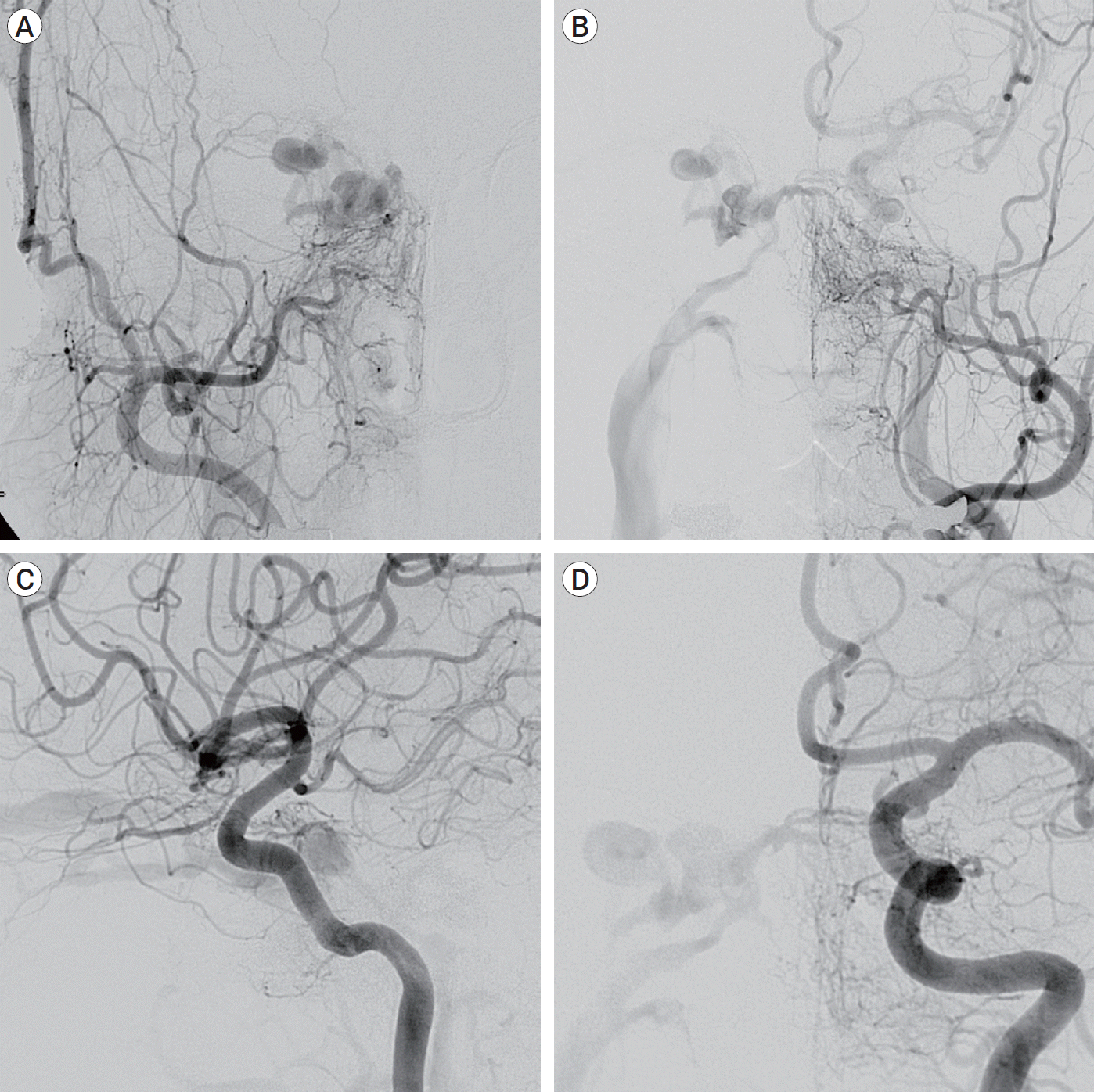 | Fig. 1.Diagnostic cerebral angiogram of a Barrow type D of complex cavernous sinus dural arteriovenous fistula. (A) Right external carotid angiogram. (B) Left external carotid angiogram. (C) Right internal carotid angiogram. (D) Left internal carotid angiogram. 
|
Under the general anesthesia, two 6 Fr femoral sheaths were placed in the femoral artery and vein, respectively. A 5 Fr angiocatheter was placed in proximal segment of the left ECA for selective control angiograms. A 6 Fr guiding catheter (Envoy; Cordis Endovascular, Miami Lakes, FL, USA) was placed in the right jugular vein and, a microcatheter (Rebar 10; Covidien/ev3, Irvine, CA, USA) was advanced over the micro-guidewire (Agility 10 standard; Cordis Endovascular, Miami Lakes, FL, USA) into the intercavernous sinus upto the fistula point. After preparation of catheter by using 0.3 mL of dimethyl sulfoxide (DMSO), under real-time road mapping, 0.4 mL of Onyx-18 (Covidien/ev3, Irvine, CA, USA) was injected by using ‘plug and push’ technique on 3 separate occasions. Onyx was well penetrated into the mural channel through the intercavernous sinus, and refluxed to distal segment of multiple fine feeders originating from the contralateral ECA and ICA (
Fig. 2A). Left ECA and ICA angiography showed no pathologic flow through the venous sinus at arterial phase. After that, a microcatheter (Prowler Select PLUS; Cordis Endovascular, Miami Lakes, FL, USA) was introduced into the cavernous sinus via the IPS to obliterate pathologic segment of cavernous sinus. The fistula was embolized by using 8 interlocking detachable coils (IDC; Boston Scientific Japan Co., Tokyo) and 5 Tornado coils (Cook, Bloomington, IN, USA). During the procedure, microcatheter was kicked-back due to densely packed coil mass, and could not be introduced into the coiled CS deeply enough to insert additional coils. Follow-up angiography revealed, however, residual shunt could not be negligible. The tip of a microcatheter could be placed into the coil mass at the juxtadistal to the junction of the coiled CS and IPS. Considering a use of liquid embolic materials, an estimated volume of required liquid embolic material was 3 ml or more to obliterate the residual shunts, we decided to use NBCA (Hystoacryl; B. Braun, Melsungen, Germany). We repeatedly checked flow patterns through the coiled CS with various injection rates via a microcatheter introduced into the fistula under the real-time road mapping. After preparation of microcatheter by using 4 mL of 5% dextrose, 3.8 mL of a 25:75 mixture of NBCA and lipiodol was injected at the rate estimated by multiple flow checks. NBCA was well penetrated into the coiled sinus, and refluxed to distal segment of multiple fine feeders originating from the ipsilateral ECA, ICA, and proximal segment of the right SOV (
Fig. 2B). Distal migration of NBCA was not observed during the procedure. The microcatheter was retrieved immediately after completion of NBCA injection.
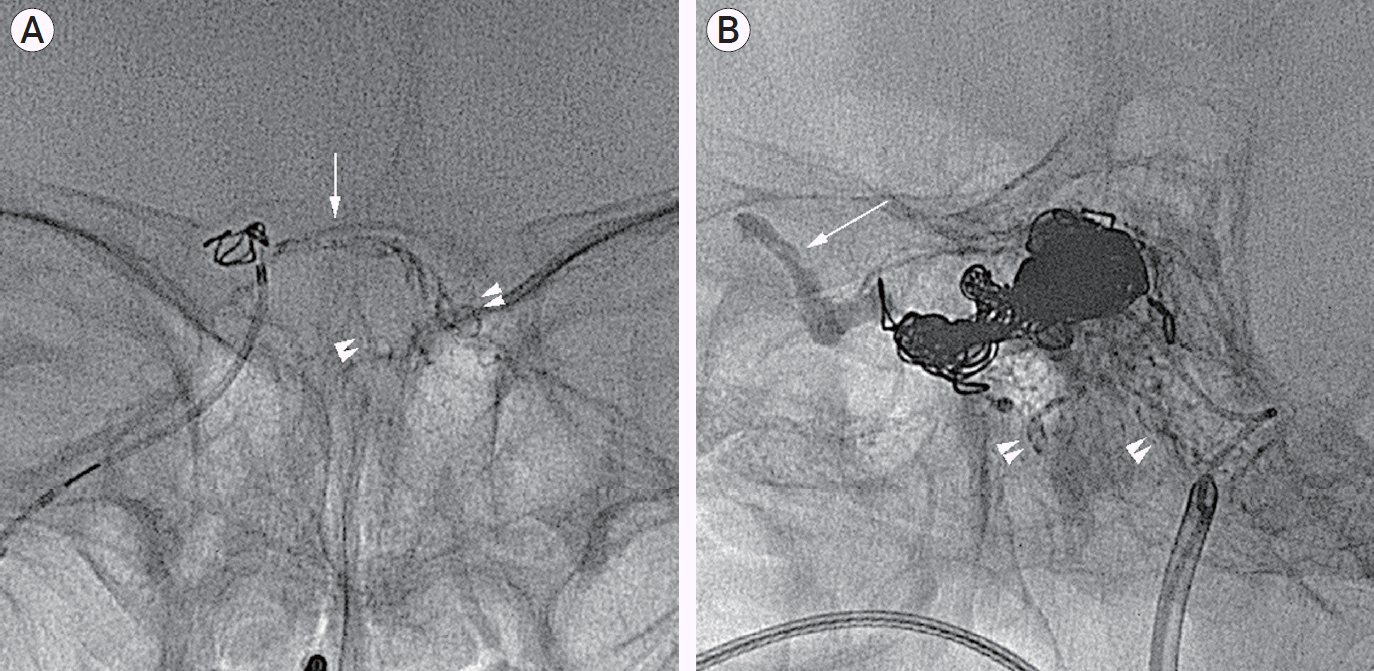 | Fig. 2.(A) Native image demonstrating an Onyx cast in the intercavernous sinus (arrow) and multiple fine feeders originating from the left ICA and ECA (arrowheads). (B) Post-embolization native image (working view) showing coil mass in the pathologic cavernous sinus, and NBCA casted in the right superior ophthalmic vein (arrow) and multiple fine feeders (arrow heads) originating from the right ECA and ICA. 
|
Complete obliteration of the fistula without residual shunt was achieved without periprocedural complications (
Fig. 3). At 4-month clinical follow-up, exophthalmos and third nerve palsy were completely recovered. Follow-up angiography was performed at 24 months after the procedure, and revealed persistent occlusion of the fistula.
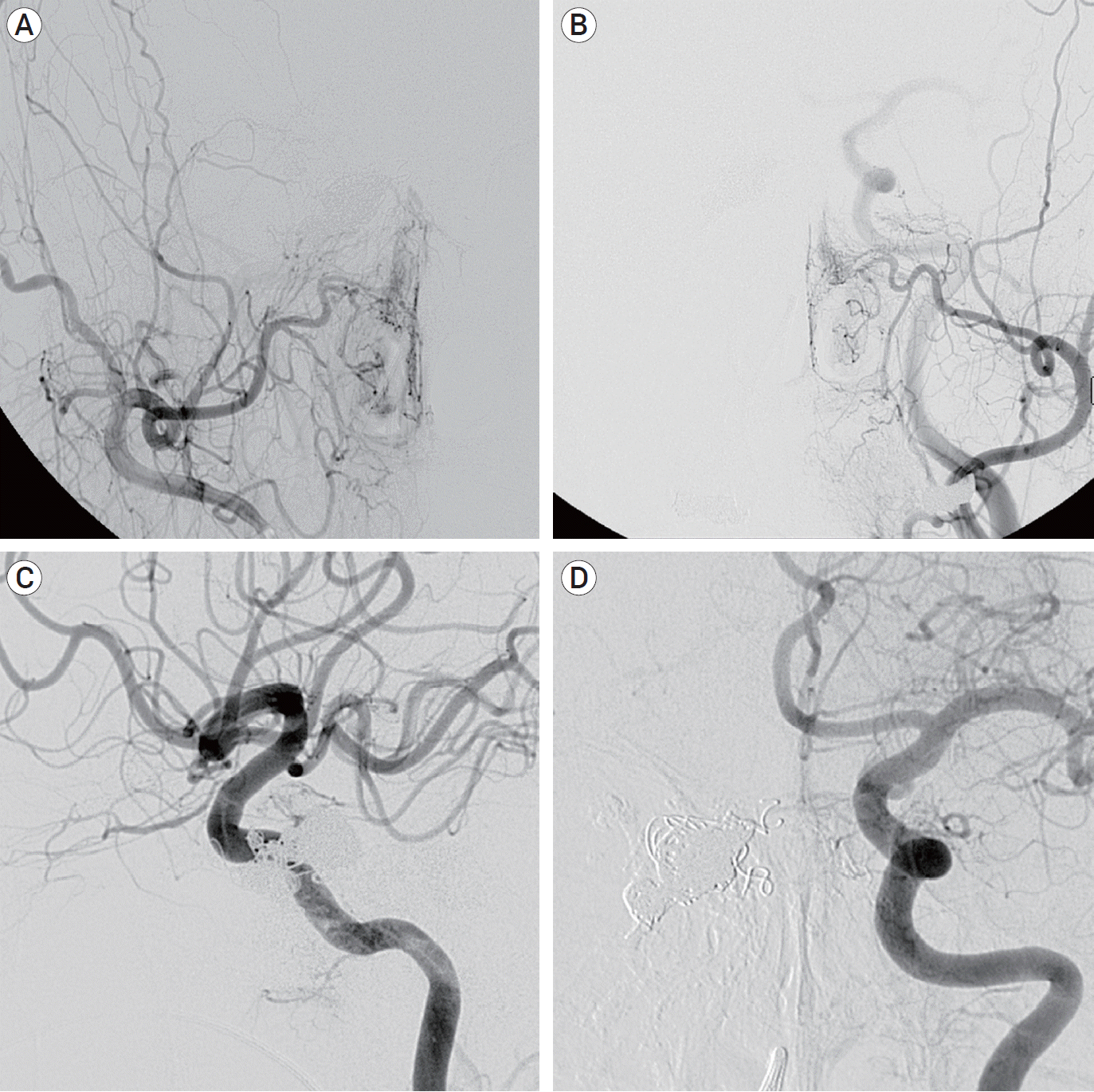 | Fig. 3.Post-embolization of the fistula without residual shunt. (A) Right external carotid angiogram. (B) Left external carotid angiogram. (C) Right internal carotid angiogram. (D) Left internal carotid angiogram. 
|
Go to :

DISCUSSION
Coil embolization of pathologic venous segment via transvenous approach to treat dAVF seems to be an indirect method. Packed coils in venous sinus promote thrombus formation, and obliterate pathologic shunt flow into the venous sinus. It could not provide direct occlusion of mural channels. Liquid embolic materials (e.g. NBCA and Onyx) can directly penetrate into mural channels via transarterial or transvenous approach, and pathologic shunts could be obliterated. Because Onyx had cohesive properties that allow controlled injection, it seems to be more favorable to obliterate dAVF through transarterial and/or transvenous approach than other liquid embolic materials. Accordingly, some authors have reported good results in treating dAVF by using Onyx [
1,
4,
7]. In this case, we achieved complete obliteration of complex fistulas recruited in the intercavenous sinus via multiple fine feeders originating from contralateral ECA and ICA using transvenous injection of Onyx-18. Compared with Onyx, NBCA has been considered not to be suitable for transvenous injection for CS-dAVF due to its adhesiveness. In addition, there is a relatively higher potency of retrograde spillage into the draining vein and ICA, distal migration, and inappropriate occlusion of pathologic segment owing to early crystallization during injection of NBCA [
2,
4,
7].
We achieved complete obliteration of pathologic CS by using intravenous NBCA injection after coil embolization. In this case, it was impossible to wedge a microcatheter within IPS or CS to achieve flow arrest of targeted vessel. Hereby, there were some risks to use NBCA. One of the potential risks was dispersion of the agent into the arterial system through the feeders originated from the ECAs and ICAs. Other potential problems may be reflux of NBCA via IPS, and uncontrolled distal migration of NBCA through SOV, superficial middle cerebral vein, or sphenoparietal sinus. Considering dispersion into the arterial system, it seems to be unlikely due to arterial blood pressure and the opposite direction of arterial feeders’ flow [
2]. In addition, coil mass in pathologic CS also act as a nest for NBCA, and it prevent retrograde spillage of NBCA via arterial feeders, venous system drained into the CS, IPA and uncontrolled distal migration through SOV [
4]. Another potential risk was early crystallization of NBCA prior to complete obliteration of pathologic CS due to insecurity of adequate stagnant nonionic environment. We could overcome these limitations via coil mass in pathologic CS. Coil mass reduced shunt flow and might extent stay of 5% dextrose in the pathologic CS [
6]. Consequently, in addition to 10 mL of abundant irrigation of 5% dextrose and 25% of slow-setting glue, coil mass might prevent early polymerization of NBCA in the vast space of pathologic CS. During injection of NBCA, glue was continuously infiltrated into coiled pathologic CS as a one column to proximal segment of SOV, and refluxed into distal segment of multiple fine feeders. In complex CS-dAVF, NBCA seems to be an additional option to obliterate pathologic CS after coil embolization. In comparison with Onyx, NBCA is cost-effective, and DMSO-related complications could be avoided.
Even though flow control achieved by using coils, however, multiple injection of NBCA would be impossible in most cases, operator should decide appropriate concentration of NBCA and injection rate by multiple testing injections under real-time road mapping using various injection rates and different concentration of contrast media.
Go to :

CONCLUSIONS
Comparing coil embolization, injection of liquid embolic material via transvenous approach could lead to direct obliteration of mural channels in case of CS-dAVF. In complex CS-dAVF, NBCA seems to be feasible option to obliterate pathologic CS, especially in a case of incompletely obliterated complex CSdAVF after transvenous coil embolization.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download