Abstract
Objective
Pulsatility index (PI) is a parameter calculated by transcranial Doppler sonography (TCD), which is commonly used for patients with subarachnoid hemorrhage or ischemic stroke. However, we performed a retrospective analysis of patients with acute spontaneous intracerebral hemorrhage (ICH) to assess the function of TCD, particularly the PI.
Methods
This study involved a total of 46 patients with acute ICH who received treatment at a single center between May 2013 and December 2014. Medical records of baseline characteristics, except for the modified Rankin scale, were obtained at initial evaluation in the emergency room, and TCD was used to calculate middle cerebral artery flow velocity (MFV) and PI at admission (baseline), 24 h, and 7 days. The PI and MFV values on the affected middle cerebral artery were compared with those on the contralateral side. Linear regression analysis was used for statistical analyses (SPSS 21.0, IBM Corp., Armonk, NY, USA).
Go to : 
Acute spontaneous intracerebral hemorrhage (ICH) is a severe type of stroke and is one of the most significant complications of hypertension. Patients can be predisposed to hematoma enlargement owing to persistently marked blood pressure (BP) elevation. BP reduction is commonly practiced in patients with spontaneous ICH to prevent hematoma enlargement. However, the relationship between BP changes and cerebral hemodynamics remains uncertain. Thus, there have been many controversies and suggestions regarding the initial management of BP in the acute phase of ICH [2,5,6,9,11,17,18,20,21,25].
Transcranial Doppler sonography (TCD) is a non-invasive, easily available diagnostic modality that provides information about the relationship between cerebral autoregulation and functional regulation of cerebral microcirculation in cerebral blood flow (CBF). The pulsatility index (PI) of cerebral vessels measured using TCD has been suggested to reflect the stiffness of vessels and distal vascular resistance for CBF [10,12,14,19,27]. However, appropriate evaluation of the potential role of PI using TCD in acute spontaneous ICH is still lacking.
This study aimed to explore the role of PI and its correlation with blood flow in the middle cerebral artery (MCA).
Go to : 
The research was performed on 46 patients with dedicated stroke services at our center. The protocol was reviewed by the agency review committee and introduced as a standard practice. The protocol was introduced as a standard practice after review by the Institutional Review Board.
In this study, 46 patients represented the retrospective methods used in patients treated within 6 h post-stroke. All patients were admitted to our center between May 2013 and December 2014. For inpatients with unknown timing of onset, the last time the patient was intact was defined as the onset. The inclusion criteria were as follows: (1) No surgical removal of the hematoma was performed; (2) supratentorial ICH, which is defined as sudden bleeding in the brain parenchyma located in the supratentorial region, which may extend to the ventricles and/or subarachnoid space, identified by computed tomography (CT) scan and clinical history; (3) neurological status of seven points or higher on the Glasgow coma scale (GCS). The following exclusion criteria were applied: (1) the onset time of symptoms could not be reliably evaluated; (2) fluctuation of neurological symptoms or progressively increasing volume of hematoma was observed; (3) previously diagnosed intracranial neoplasm, trauma, intracranial aneurysms, arteriovenous malformation had been documented; or (4) ICH was located in the ventricle only, or the infratentorial space, such as the cerebellum or brainstem; (5) any history of coagulopathy or bleeding diathesis or medications promoting coagulopathy; and (6) any history of myocardial infarction, congestive heart failure, kidney failure, or blood sugar levels below 50 mg/dL or over 300 mg/dL.
The target BP of the treatment group was systolic BP (SBP) <140 mmHg and diastolic BP (DBP) <100 mmHg for the first 24 h after symptom onset. The treatment was initiated using IV nicardipine (10 mg/h) if necessary, and when the target BP was not reached, the dosage was increased by 2.5 mg/h every 15 min to the maximum allowable dose (15 mg/h), or until nicardipine side effects restricted the use of the regimen till the full dose of medication was reached. Oral nicardipine was not used during BP maintenance.
The protocol was made available in the form of preprinted order sheets and, after the initial assessment, was inserted into the chart as routine orders. BP was tracked for 7 days using an automated cuff inflator or an intra-arterial catheter at the physician’s discretion. If the BP decreased below a specific level and the symptoms corresponding to or likely worsened due to hypoperfusion, administration of nicardipine was discontinued and further management was performed followed by the attending physician.
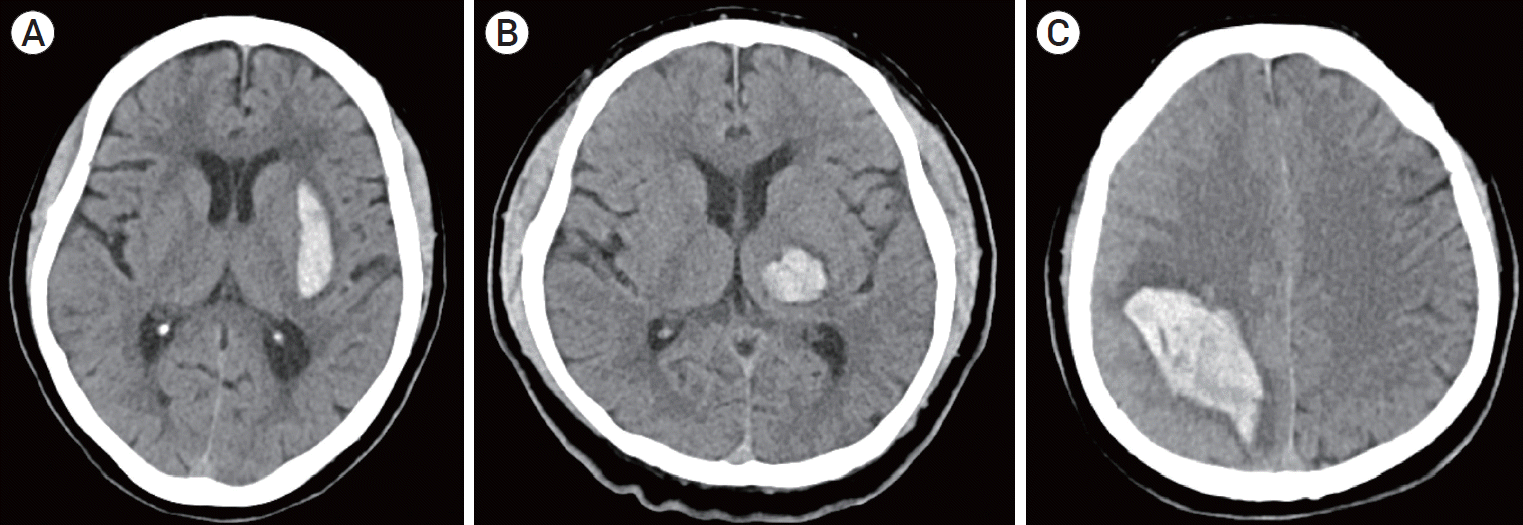
Data for all patients treated following the hypertension treatment protocol were recorded using a standard questionnaire. From the medical records of eligible patients, we obtained relevant information, including age, sex, and the existence of any of the following risk factors before the occurrence of ICH: previous cerebral stroke, heart disease, hypertension, and diabetes mellitus. The initial GCS score was used to evaluate the neurological condition at presentation, as recorded in the initial examination. The location of ICH was confirmed using CT and recorded for all patients, including the basal ganglia, thalamus, and lobar (Fig. 1). The functional outcome of all patients was assessed 6 months after treatment using the modified Rankin scale (mRS).
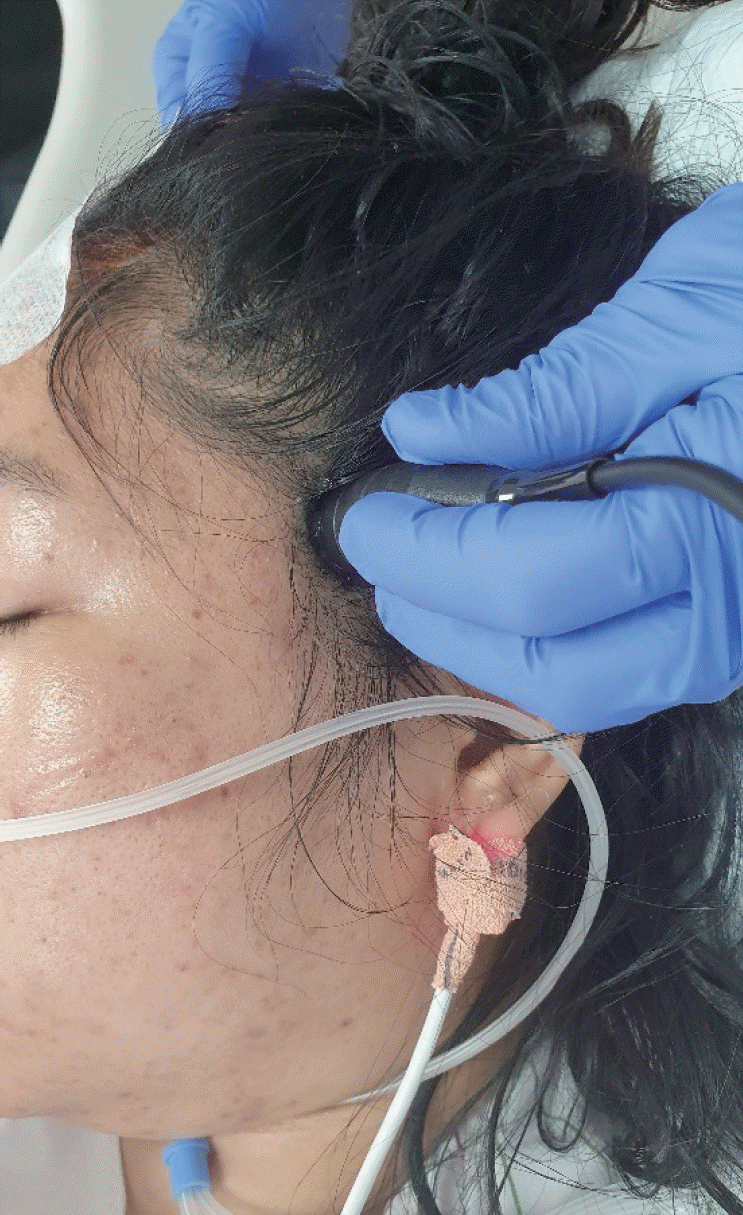
The patients were positioned on a standard hospital bed in a 30˚ recumbent position. TCD monitoring was performed using digital equipment (Spencer Technologies, Seattle, WA, USA) with Power M-ModeTM technology. A 2-MHz probe was used to instigate the proximal segments of the MCA over a depth of 66 mm. The system was used in conjunction with the head frame of the Spencer Technologies Marc series for long-term monitoring. Spontaneous fluctuations were recorded for 10 min after stabilization of the hemodynamic parameters.
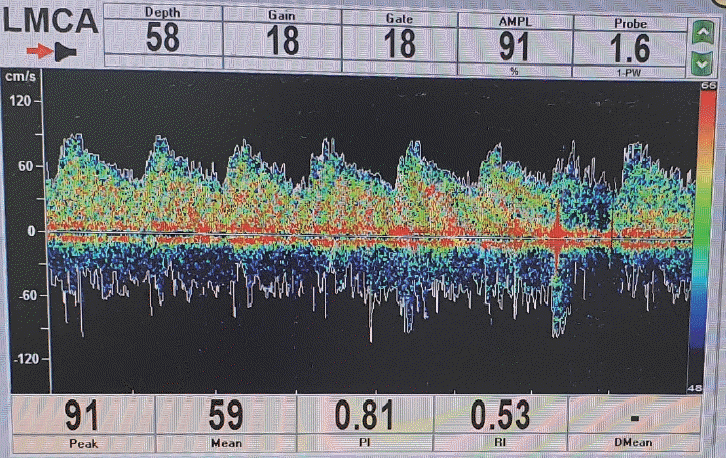
The following parameters were continuously recorded: (1) MCA flow velocity (MFV), the value was obtained when the Doppler probe was placed on the temporal region of the head insonating MCA (Fig. 2), and the mean MFV was calculated directly by the modality (Fig. 3); (2) PI calculated directly by the equipment based on Gosling’s formula ([peak systolic CBF velocity–end-diastolic CBF velocity]/mean CBF velocity) (Fig. 3). The PI and mean MFV were recorded for the ipsilateral affected MCA and contralateral normal side. In all patients who had asymmetric ICH distributions determined using CT scans, a Doppler probe was placed on the side with a greater volume of hematoma, and the parameters of the ipsilateral MCA were evaluated.
Statistical analysis was performed using linear regression analysis (SPSS 21.0, IBM Corp., Armonk, NY, USA). Continuous variables are expressed as the mean±-standard deviation (range), and categorical variables are expressed as the number of patients (percentage). Statistical significance was set at p<0.05.
Go to : 
The registry included 46 patients. This included 21 men (45.6%) and 25 women (54.4%); the average age was 64±12.5 years. There were previously known risk factors, including hypertension in 42 patients who required antihypertensives and other risk factors, including coronary heart disease (five patients), previous cerebral stroke (nine patients), diabetes mellitus (five patients), and end-stage renal disease (two patients). The average volume of hematoma was 17. 6±18.5 mL (most of small and medium-size). The average GCS score at admission was 13.1±2.1. In most cases, the size of the hematoma was small to medium (average hematoma volume 17.6±18.5). After 6 months of treatment, the mRS score distribution was mRS 0, 1; mRS 1, 15; mRS 2, 16; mRS 3, 9; mRS 4, 3; mRS 5, 1; and mRS 0, 1 (Table 1). Hourly BP records for treatment were recorded for all patients. The average initial SBP and DBP values at the baseline (admission) were 160.8±30.5 mmHg and 98.5±20.4 mmHg, respectively. Following the protocol, administration of nicardipine HCl reduced the average SBP and DBP (124.6±15.8, 122.4±13.4 [24 h] and 72.6±11.5, 74±10.8, and 7 days, respectively) (Table 2). TCD monitoring was performed at baseline (admission), 24 h, and 7 days after the occurrence of ICH. CBF, represented by the average MCA velocity, was noted for every patient. The average MCA velocity in patients treated with nicardipine HCl was not significantly decreased on the affected side (50±7.8 cm/s) compared to CBF on the unaffected side (44±13.3 cm/s, p=0.51) at admission. Additionally, the average MCA velocity at 24 h and 7 days of outbreak was not decreased significantly compared to the CBF between the affected (11.1±0.25 cm/s) and normal sides (Table 2). The PI, a reliable predictor of functional clinical outcomes, was recorded in the affected MCA (11.1±0.25 cm/s) compared to CBF on the contralateral normal side (10.1±0.4 cm/s, p=0.22), making no significant changes (at admission). The 24-h and 7-day PI results of the the affected and contralateral MCA were not significantly different. Grouping results by affected and normal unaffected MCA showed higher PI and lower MFV on the affected side compared to the normal contralateral side on the result using TCD. However, these differences were not statistically significant, and as time changed after the occurrence of ICH, the variables of PI and MFV of the affected MCA returned to their original values (Table 2). The distribution of the relationship between variables and PI is shown in Table 3. As suggested by the results of the linear regression analysis, while mRS at 6 months after treatment and PI had a significantly positive correlation (R=0.846, p=0.002), other variables (sex, age, GCS, intraventricular hemorrhage, hematoma size, risk factors) were negatively correlated (p>0.05).
Baseline characteristics of study patients (n= 46)
Hemodynamic characteristics; BP, MFV & PI
Relations between variables & pulsatility index (linear regression model analysis)
Go to : 
The claim for treatment of hypertension in patients with acute ICH is based primarily on concerns that BP reduction induces CBF reduction. Several previous studies have suggested that a temporary reduction in CBF in the peripheral and distal regions of the hematoma is probably induced by compression of adjacent microvascular lines. Additional assumptions have been made that local ischemia and acidosis damage self-regulation in the region around the hematoma. Thus, theoretically, a decrease in systemic BP can induce further impairment of blood flow and cause decreased CBF and ischemia. Lowering systemic BP can also lead to auto-regulated vessel dilatation of the cerebral vessels and adversely increase ICP. The increased BP can also be the result of the Cushing-Kocher reaction to maintain cerebral perfusion. Under normal conditions, extensive changes in BP have an insignificant influence on the CBF. This automatic regulation of CBF relies on change in the diameter of the resistance arterioles, which is a reaction to changes in cerebral perfusion pressure (CPP). As CPP falls, the arterioles expand, reducing resistance and maintaining CBF. This auto-regulating vessel dilation has a limit, and CBF starts to fall when it reaches its limit [2,3,5,6,13,15,16,23,25].
The TCD enables hemodynamic evaluation of the MCA region, which predominantly reflects cerebral cortical arterioles. By applying TCD, the presence of mass lesions can lead to deformation of the affected MCA. In this circumstance, the CBF velocity signal from the MCA still reflects downstream arteriolar resistance. Significant deformation can lead to compression or torsion of the affected MCA, resulting in a stenotic signal with a turbulent, elevated CBF velocity. The PI was obtained based on the Gosling formula. PI is routinely used to evaluate resistance to intracerebral blood flow in diagnostic TCD studies of patients with stroke. Arterial wave reflections in low-resistance vascular beds, such as the brain, are likely to be insignificant. Therefore, CBF pulsatility is likely to be primarily determined by wave reflections reflected from other vessel beds with high resistance. Thus, it was hypothesized that while CBF decreases, PI increases due to the elevation of cerebral artery stiffness, pressure pulsatility, and wave reflection [4,7,8,22,25,26,28].
Cerebral stroke, which includes ICH and ischemic lesions, has long been considered to result from mechanical insults to cerebral microcirculation, particularly affected by hemodynamic pulsatility. One previous study has shown that in patients with ischemic stroke, the diffuse intracranial arterial disease is frequently found and can be reliably predicted by abnormal cerebral parameters on TCD. In particular, under the presence of high PI, low MFVs have been reported to be associated independently with diffuse intracranial arterial disease when assessed using invasive angiogram [1,24].
Another study found that in this specific TCD pattern, the severity of diffuse intracranial arterial disease, indicated by the values of PI and MFV measured using TCD, is an independent prognostic factor for recurrent vascular events in patients with acute ischemic stroke [28]. However, a different study that evaluated the prognostic value of PI in acute ICH found that the PI value of the unaffected hemispheres was correlated with patient mortality. Therefore, we concluded that PI may be a predictor of mortality in patients with acute ICH [15].
The acute appearance of a new mass (ICH) temporarily impairs intracerebral blood flow in the affected region of the brain. As ICP increases, CPP decreases, diastolic and mean CBF decreases, and PI increases. Our results in patients with spontaneous ICH showed higher PI and lower MFV on the affected side throughout the course of the study, even at baseline levels, before administration of nicardipine HCl. Compensation of blood flow, which restores cerebral perfusion in the reversible tissues surrounding the hematoma on the affected side of the brain, may explain the lower MFV measured in this study, as described for functional neuroimaging in the penumbra area; however, in our case, it was during the acute and subacute stages of cerebral ischemia. The previously observed lower MFV in the affected MCA was identified using TCD. However, we can assume that diseased cerebral tissues near the hematoma persist, requiring re-perfusion. Further, PI is an indirect measurement of distal microvascular resistance that can be affected by the local mass effect produced by the hematoma. In this study, the mass effect induced by hematoma and perihematomal edema may not be the result of the high PI observed in the affected hemisphere. A higher PI on the affected MCA may indicate preserved pulsatility and cerebral hemodynamics in the affected hemisphere. Our findings demonstrate that autoregulation of the brain sustained in the acute phase of ICH temporarily delines, and is restored secondarily after nicardipine HCl administration, which is related to higher PI and lower MFV. In a previous study [9]. it was mentioned that the brain was able to maintain autoregulation in the appearance of small- to medium-sized hematomas. However, even with a larger volume of hematoma, MFV was consistently depressed and PIs were elevated, while the PI ratio showed no relationship with the hematoma volume.
PI strongly reflects the increased flow resistance downstream, particularly in patients with cerebral small vessel disease and diffuse atherosclerosis caused by chronic hypertension. Thus, TCD results can be influenced by other pathophysiological correlations, such as small and large-vessel atherosclerosis. This can also elucidate why there was a significant link between the TCD resistance index, such as arterial hypertension, and PI, as the former plays a critical role in the development of cerebral small vascular disease. The PI also rises when the measured vessel segment has a barrier. This is likely to be associated with increased ICP, which is expected in the early stages of ICH. Previous studies have found a negative correlation between MCA blood flow velocity, particularly the end-diastolic blood flow velocities, and clinical scores obtained during acute and chronic stages of acute stroke [1]. The link between end-diastolic blood velocity and favorable functional outcomes is consistent with recent studies. Unlike the peak systolic velocity, the increase in end-diastolic velocity is related to early neurological improvements and favorable functional outcomes. PI measured using TCD in the intracranial artery can act as a surrogate parameter for ICP. It also serves as a reliable predictor of functional outcomes in patients with ICH. PI, which is one of the indices measured using TCD, can play a role in predicting positive clinical and functional outcomes. In this sense, we suggest that this test should be performed in all patients with ICH.
There are several limitations to the design of our study. The first limitation was related to patient selection criteria. This included small- to medium-sized ICH and relatively good GCS scores. Patients with large-sized ICH were excluded, and the results of this study may not accurately reflect ICH. In addition, individuals with large ICH may have increased ICP correlated with a secondary elevation of systemic BP. Since cerebral perfusion is the difference between systemic BP and ICP; therefore, a drop in systemic BP may induce impaired cerebral perfusion. This can be worsened in patients with hypertension. Compared to smaller hematomas, larger hematomas are correlated with lower intracranial conformity and higher intracranial pressure. The lower the compliance, the stiffer the affected brain tissues, which may damage the fast-responding myogenic response. Dynamic brain autoregulation deterioration was greater in the higher ICP group than in the lower ICP group. As aforementioned, IICP has been thought to be related to PI; however, in this study, the relationship between the volume of hematoma and PI was relatively low (Table 3). Although several factors are related to IICP, preexisting brain atrophy, presence of encephalomalacia, and location and volume of hematoma are factors that also affect ICP. This might be as the number of patients with large-sized ICH was relatively small compared to cases with small- to medium-sized ICH, as most of the patients with large-sized ICH underwent surgical removal of hematoma, which does not meet the inclusion criteria. Therefore, future studies should target a larger number of patients to include a sufficient number of patients with large-sized ICH. The second limitation of this study is that the number of cases (46 cases) is relatively small and is not assumed to be sufficient to conclude the importance of PI as a diagnostic modality. Moreover, the existence of unknown and confusing variables that were not accounted for in the final result cannot be excluded. Additionally, it is not possible to exclude the possibility that the dynamic cerebral autoregulation in the ICH group had already been damaged before bleeding due to underlying vascular risk factors such as hypertension. For patients who have not been treated for hypertension, a static cerebral autoregulatory curve is known to shift to the right. Thus, our findings on ICH patients’ bilateral dynamic cerebral autoregulation impairment might be caused by a patient’s history of hypertension. Third, the PI was estimated from the amplitude of the CBF waveform for the mean value. This method allows the direct comparison of CBF pulsatility between individuals with different CBF levels; however, further studies should determine whether the CBF pulsatility amplitude is more clinically related to brain structure and function. Finally, some well-known confounding factors of TCD for evaluating cerebral autoregulation are difficult to control outside the intensive care unit settings. Severe intracranial or extracranial arterial stenosis and underlying conditions (asymptomatic infarction, diabetes, and chronic hypertension) can also confuse the evaluation of cerebral autoregulation.
Go to : 
This study provides evidence that PI is an independent determinant of acute spontaneous ICH. Specifically, based on the results of our analysis, we conclude that increased PI can serve as a reliable predictor of functional outcomes in patients with ICH. If abnormal TCD results can cause suspicion in patients with ICH, this information can be used to guide these patients to specific diagnostic assessments and considerations for treatment options. Further research is needed to investigate whether lowering BP affects CBF flow dynamics on a larger, more controlled, and more randomized basis. However, our data can be helpful for designing such trials and provide useful guidelines for reducing arterial pressure in patients with ICH.
Go to : 
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Go to : 
REFERENCES
1. Alexandrov AV, Tsivgoulis G, Rubiera M, Vadikolias K, Stamboulis E, Molina CA, TUCSON Investigators, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomized pilot trial. Lancet Neurol. 2008; May. 7(5):391–9.
2. Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, INTERACT Investigators, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomized pilot trial. Lancet Neurol. 2008; May. 7(5):391–9.
3. Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010; Nov. 41(11):2697–704.
4. Barlinn K, Kolieskova S, Shahripour RB, Kepplinger J, Boehme AK, Siepmann T, et al. Increased pulsatility of the intracranial blood flow spectral waveform on transcranial Doppler does not point to peripheral arterial disease in stroke patients. J Stroke Cerebrovasc Dis. 2015; Jan. 24(1):189–95.

5. Bullock R, Brock-Utne J, van Dellen J, Blake G. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surg Neurol. 1988; Feb. 29(2):101–7.

6. Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998; Jun. 29(6):1160–6.

7. Fülesdi B, Réka Kovács K, Bereczki D, Bágyi P, Fekete I, Csiba L. Computed tomography and transcranial Doppler findings in acute and subacute phases of intracerebral hemorrhagic stroke. J Neuroimaging. 2014; Mar-Apr. 24(2):124–30.

8. Han SW, Song TJ, Bushnell CD, Lee SS, Kim SH, Lee JH, et al. Cilostazol decreases cerebral arterial pulsatility in patients with mild white matter hyperintensities: subgroup analysis from the Effect of Cilostazol in Acute Lacunar Infarction Based on Pulsatility Index of Transcranial Doppler (ECLIPse) study. Cerebrovasc Dis. 2014; 38(3):197–203.

9. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; Jul. 46(7):2032–60.

10. Heyer EJ, Mergeche JL, Connolly ES Jr. Middle cerebral artery pulsatility index and cognitive improvement after carotid endarterectomy for symptomatic stenosis. J Neurosurg. 2014; Jan. 120(1):126–31.

11. Hwang SK, Kim JS, Kim JH, Hong CK, Yang KH. Antihypertensive treatment of acute intracerebral hemorrhage by intravenous nicardipine hydrochloride: prospective multi-center study. J Korean Med Sci. 2012; Sep. 27(9):1085–90.

12. Kim DH, Choi JH, Moon JS, Kim HJ, Cha JK. Association between the severity of cerebral small vessel disease, pulsatility of cerebral arteries, and brachial ankle pulse wave velocity in patients with lacunar infarction. Eur Neurol. 2010; 64(4):247–52.

13. Kim JY, Bushnell CD, Park JH, Han SM, Im JH, Han SW, et al. Central aortic pressure and pulsatility index in acute ischemic stroke. J Neuroimaging. 2015; May-Jun. 25(3):438–42.

14. Kiphuth IC, Huttner HB, Dörfler A, Schwab S, Köhrmann M. Doppler pulsatility index in spontaneous intracerebral hemorrhage. Eur Neurol. 2013; 70(3-4):133–8.

15. Martí-Fàbregas J, Belvís R, Guardia E, Cocho D, Muñoz J, Marruecos L, et al. Prognostic value of Pulsatility Index in acute intracerebral hemorrhage. Neurology. 2003; Oct. 61(8):1051–6.

16. Nath FP, Kelly PT, Jenkins A, Mendelow AD, Graham DI, Teasdale GM. Effects of experimental intracerebral hemorrhage on blood flow, capillary permeability, and histochemistry. J Neurosurg. 1987; Apr. 66(4):555–62.

17. Oeinck M, Neunhoeffer F, Buttler KJ, Meckel S, Schmidt B, Czosnyka M, et al. Dynamic cerebral autoregulation in acute intracerebral hemorrhage. Stroke. 2013; Oct. 44(10):2722–8.

18. Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004; Jun. 35(6):1364–7.
19. Park KY, Chung PW, Kim YB, Moon HS, Suh BC, Yoon WT. Increased pulsatility index is associated with intracranial arterial calcification. Eur Neurol. 2013; 69(2):83–8.

20. Powers WJ, Zazulia AR, Videen TO, Adams RE, Yundt KD, Aiyagari V, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001; Jul. 57(1):18–24.

21. Qureshi AI, Safdar K, Weil J, Barch C, Bliwise DL, Colohan AR, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995; Oct. 26(10):1764–7.

22. Rajajee V, Fletcher JJ, Pandey AS, Gemmete JJ, Chaudhary N, Jacobs TL, et al. Low pulsatility index on transcranial Doppler predicts symptomatic large-vessel vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2012; May. 70(5):1195–206. discussion 1206.

23. Reinhard M, Neunhoeffer F, Gerds TA, Niesen WD, Buttler KJ, Timmer J, et al. Secondary decline of cerebral autoregulation is associated with worse outcome after intracerebral hemorrhage. Intensive Care Med. 2010; Feb. 36(2):264–71.

24. Sharma VK, Tsivgoulis G, Lao AY, Malkoff MD, Alexandrov AV. Noninvasive detection of diffuse intracranial disease. Stroke. 2007; Dec. 38(12):3175–81.

25. Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014; Jun. 34(6):971–8.

26. Vicenzini E, Ricciardi MC, Zuco C, Sirimarco G, Di Piero V, Lenzi GL. Effects of a single mannitol bolus on cerebral hemodynamics in intracerebral hemorrhage: a transcranial Doppler study. Cerebrovasc Dis. 2011; 32(5):447–53.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download