서론
본론
1. 혈당 조절 약제
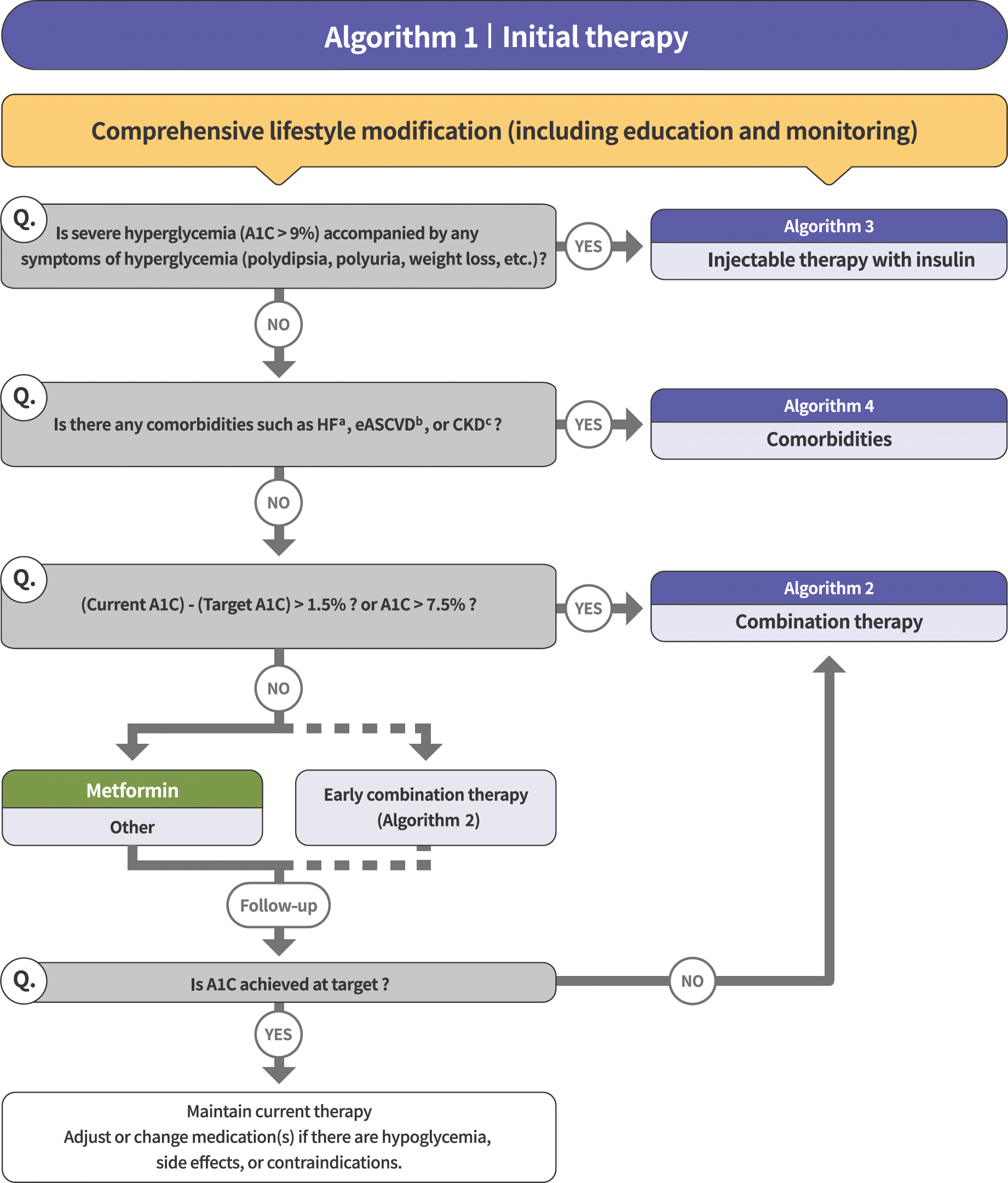 | Fig. 1.Treatment algorithm in patients with type 2 diabetes [4]. |
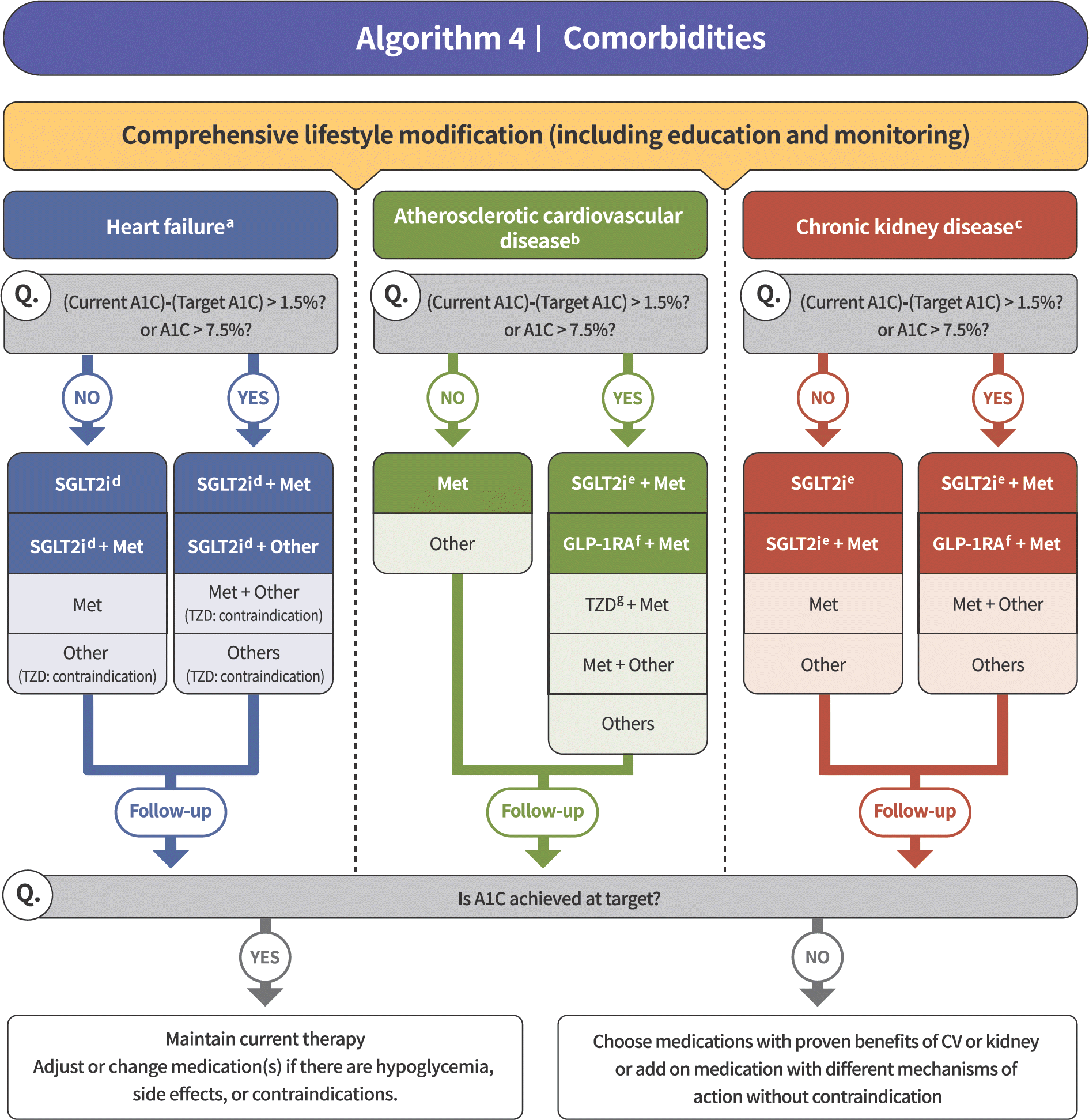 | Fig. 2.Treatment algorithm in patients with type 2 diabetes and heart failure, atherosclerotic cardiovascular disease, or chronic kidney disease [4]. |
Table 1.
EMPA-REG OUTCOME, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose trial; CANVAS, Canagliflozin Cardiovascular Assessment Study; CANVAS-R, CANVAS-Renal; DECLARE-TIMI 58, Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58; CREDENCE, Canagliflozin and Renal End-points in Diabetes with Established Nephropathy Clinical Evaluation; VERTIS-CV, eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial; CV, cardiovascular; HF, heart failure; MACE, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval; MI, myocardial infraction; NA, not available.
Table 2.
| ELIXA | LEADER | REWIND | SUSTAIN-6 | PIONEER-6 | |
|---|---|---|---|---|---|
| No. of patients | 6,068 | 9,340 | 9,901 | 3,297 | 3,183 |
| Drug | Lixisenatide | Liraglutide | Dulaglutide | Semaglutide SQ | Semaglutide oral |
| Median duration of follow-up (y) | 2.1 | 3.8 | 5.4 | 2.1 | 1.3 |
| Mean baseline A1c (%) | 7.7 | 8.7 | 7.2 | 8.7 | 8.2 |
| Baseline ASCVD/HF (%) | 100 | 81 | 31 | 72 | 85 |
| Baseline HF (%) | 22 | 18 | 9 | 24 | NR |
| MACE outcomea, HR (95% CI) | 1.02 (0.89∼1.17) | 0.87 (0.78∼0.97) | 0.88 (0.79∼0.99) | 0.74 (0.58∼0.95) | 0.79 (0.57∼1.11) |
| CV death, HR (95% CI) | 0.98 (0.78∼1.22) | 0.78 (0.66∼0.93) | 0.91 (0.78∼1.06) | 0.98 (0.65∼1.48) | 0.49 (0.27∼0.92) |
| Fatal or nonfatal MI, HR (95% CI)b | 1.03 (0.87∼1.22) | 0.86 (0.73∼1.00) | 0.96 (0.79∼1.15) | 0.74 (0.51∼1.08) | 1.18 (0.73∼1.90) |
| Fatal or nonfatal stroke, HR (95% CI)b | 1.12 (0.79∼1.58) | 0.86 (0.71∼1.06) | 0.76 (0.62∼0.94) | 0.61 (0.38∼0.99) | 0.74 (0.35∼1.57) |
| All-cause mortality, HR (95% CI) | 0.94 (0.78∼1.13) | 0.85 (0.74∼0.97) | 0.90 (0.80∼1.01) | 1.05 (0.74∼1.50) | 0.51 (0.31∼0.84) |
| HF hospitalization, HR (95% CI) | 0.96 (0.75∼1.23) | 0.87 (0.73∼1.05) | 0.93 (0.77∼1.12) | 0.86 (0.48∼1.55) | 1.11 (0.77∼1.61) |
| Renal composite outcomec | 0.84 (0.68∼1.02) | 0.78 (0.67∼0.92) | 0.85 (0.77∼0.93) | 0.64 (0.46∼0.88) | 0.64 (0.46∼0.88) |
ELIXA, Evaluation of Lixisenatide in Acute Coronary Syndrome; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; REWIND, Researching Cardiovascular Events with a Weekly Incretin in Diabetes; SUSTAIN-6, Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes; PIONEER, Peptide Innovation for Early Diabetes Treatment; ASCVD, atherosclerotic cardiovascular disease; HF, heart failure; MACE, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval; CV, cardiovascular; MI, myocardial infraction; NR, not reported.
b The risk estimates and 95% CIs for SUSTAIN-6 is for nonfatal MI (excluding fatal MI) or nonfatal stroke (excluding fatal stroke). The effect estimates for the composite endpoints of fatal or nonfatal MI and fatal or nonfatal stroke were not available in the primary manuscripts.
c The renal composite outcome reported in a recent meta-analysis was a composite of the development of macroalbuminuria doubling of serum creatinine, a ≥ 40% decline in eGFR, development of end-stage kidney disease, or death due to renal causes. For SUSTAIN-6, the renal composite was persistent macroalbuminuria, persistent doubling of serum creatinine with an eGFR < 45 mL/min/1.73 m2 need for continuous renal replacement therapy.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download