INTRODUCTION
The incidence of sweat gland carcinoma (SGC) is approximately 0.005% among all malignant epithelial neoplasms of the skin [
1]. Malignant nodular hidradenoma (MNH) was first reported by Keasby and Hadley in 1954. It accounts for 6% of malignant eccrine tumors, which in turn are found in 1:13,000 skin biopsies [
2]. Several cases have been published under various names, called MNH, clear cell hidradenocarcinoma, malignant clear cell hidradenoma, malignant clear cell myoepithelioma, and malignant acrospiroma.
They usually occur on the scalp, trunk, and proximal limbs after the fifties, and rarely occur in the distal extremities [
2]. Clinical features include solitary, firm, asymptomatic intradermal nodule, and occasionally ulcerated skin, which grows slowly over several years and then suddenly increases in size. MNH recurs in 50% of the total cases and is reported to metastasize to lymph nodes, bones, and visceral organs in 60%, finally leading to death [
1]. The pathological findings widely vary and are rarely identified, thus the probability of misdiagnosis is high [
3].
In a case report by Chang et al. [
2] in 2011, for the first time, treatment with a clear resection margin of hidradenocarcinoma on the right index fingertip was introduced. We would like to report the first case of MNH on the fingertip in Korea.
Go to :

CASE REPORT
A 55-year-old male patient came to the hospital with a painless mass that began to grow slowly from a year ago. The size suddenly increased from the last 3 weeks, and ulceration and bleeding occurred.
Clinically found firm mass of 1.5×3.0 cm and partly ulcerated lesion was observed in the distal volar pulp of the left index finger (
Fig. 1). It showed a normal range of motion and there was no numbness or tingling sensation. At the clinic, we could not found any bony anomaly on the X-ray, so ultrasound and magnetic resonance imaging (MRI) were performed. Ultrasound showed a cystic mass with solid component arising from the deep aspect, and. Possibility of hemangioma due to increased vascularity around the mass. Official reading from the radiologist confirmed that it did not show any unusual findings on MRI (
Fig. 2).
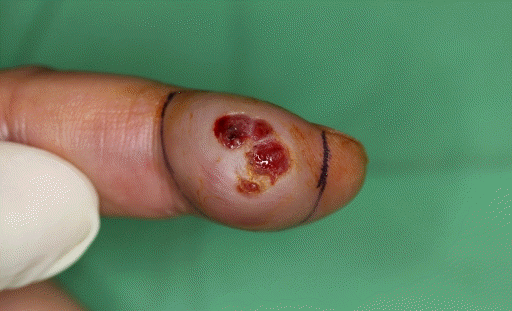 | Fig. 1.A 55-year-old male visited with a 1.5×3.0-cm firm mass. It started 1 year ago and rapidly increased in size from 3 weeks ago with central ulceration and bleeding. 
|
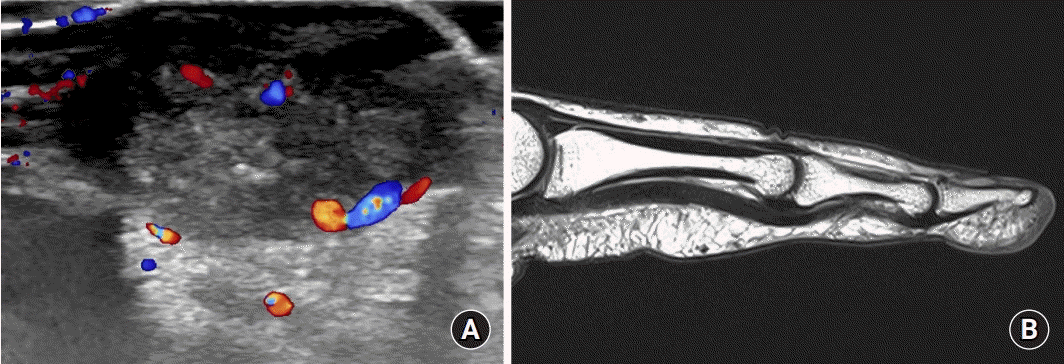 | Fig. 2.(A) Possibility of hemangioma due to increased vascularity around the mass on ultrasonography. (B) Official reading from the radiologist confirmed that it did not show any unusual findings on magnetic resonance imaging. 
|
According to benign origin impression, we had designed the skin with an 1-mm margin of ulceration edge with extending skin incision. The surgical findings showed well-demarcated mass without adhesion to the surrounding tissues. It was located subcutaneously, and a pale yellow 1.5×3.0-cm mass was removed (
Fig. 3).
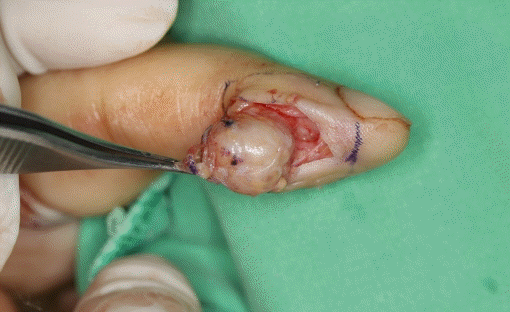 | Fig. 3.During the first operation, the firm solitary mass was removed with the stalk. 
|
Primarily closed wound healed well without any complication. According to pathologic findings, the encapsulated tumor was situated and circumscribed in the dermis. Nodular mass showed solid and cystic components. The tumor was connected to overlying epidermis and extended into the subcutaneous fat. It also consisted of solid nest showing extensive necrosis, which were surrounded by a hyalinized stroma. The tumor cells are predominantly polygonal or fusiform cells with clear or pale staining cytoplasm containing a large vesicular nucleus. Some areas appear as basaloid cells or eosinophilic cytoplasm. Intracytoplasmic lumens or duct-like structures represent ductal differentiation. Nuclear pleomorphism is apparent, and so is hyperchromatism. Mitoses are very conspicuous (
Fig. 4).
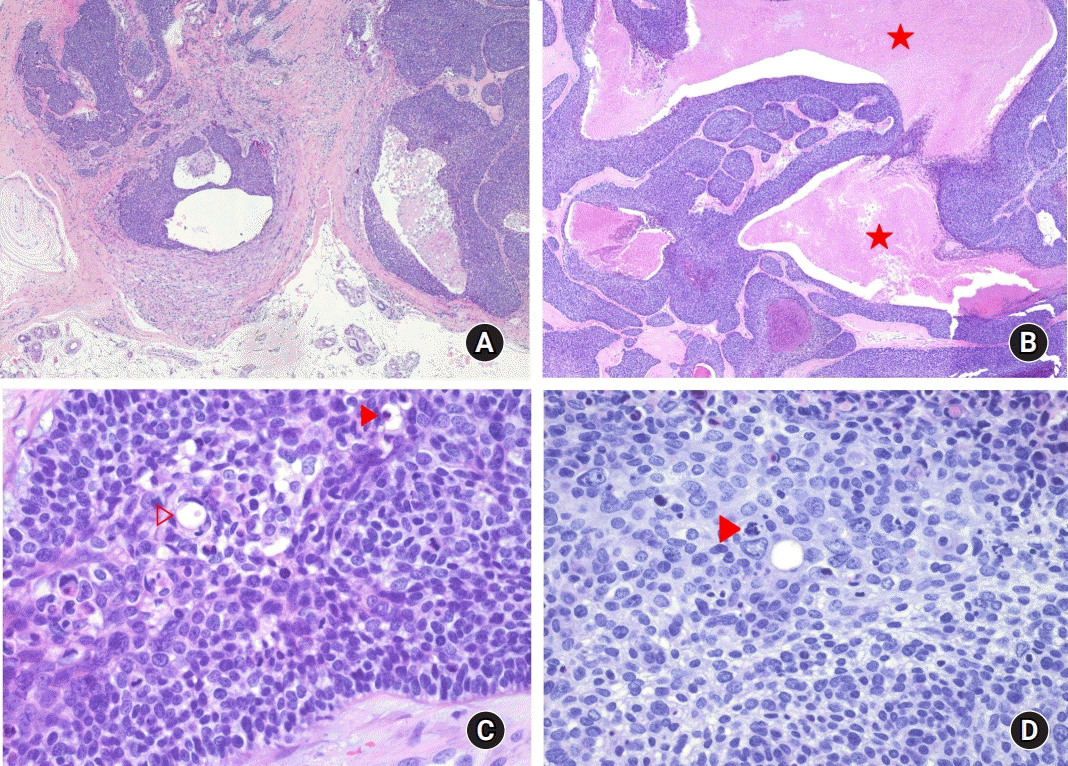 | Fig. 4.(A) Low power view show circumscribed tumor nodule and cystic areas surrounded by a hyaline stroma (H&E stain, ×10). (B) The tumor shows extensive necrosis (asterisks) (H&E stain, ×40). (C) Duct-like structure (empty arrowhead) and cytoplasmic lumen (solid arrowhead) are evident. The tumor cells have pleomorphic and hyperchromatic nuclei (H&E stain, ×400). (D) Mitotic figures (solid arrowhead) are easily found (H&E stain, ×400). Nucleoli are prominent. 
|
Based on the pathology findings, we diagnosed the MNH and requested the radiologist for the re-read of MRI findings. A tiny bone erosive lesion at distal phalanx was identified on MRI.
For further oncological evaluation, the patient was referred to the cancer center. Positron emission tomography-computed tomography (PET-CT) was taken and no distant metastasis was found. To remove the residual tumor that might remain, radical amputation was performed at the level of the distal interphalangeal joint (
Fig. 5).
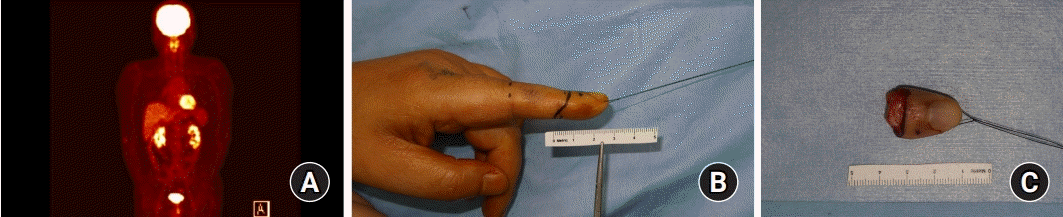 | Fig. 5.(A) No distant metastasis was observed on positron emission tomography-computed tomography. (B, C) Radical amputation was performed due to bone erosive lesions of the distal phalanx found on magnetic resonance imaging re-reading. 
|
Wound healed without any specific complication 1 year after the surgery and there was no evidence of metastasis as a result of periodic follow-up (
Fig. 6).
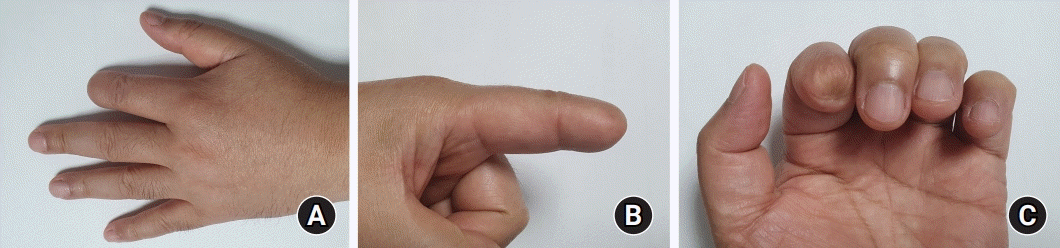 | Fig. 6.No specific complaints and no evidence of metastasis after 1 year. 
|
Written informed consent was obtained for publication of this case report and accompanying images.
Go to :

DISCUSSION
Malignant hidradenoma is a rare malignant tumor that differentiates from an eccrine gland present at the distal end of the sweat gland [
2]. In 2011, Chang et al. [
2] reported the first case of MNH on the fingertip. This is the first case report of MNH at the fingertip in South Korea.
It is very difficult to make a clinical diagnosis of MNH due to the rarity of the disease itself, controversial and complex terminology, and various systematic findings. Clinically, it mainly occurs as a solitary mass on the extremities, on the head or on the trunk, and as an intradermal nodule with a size of 0.5 to 2 cm [
4]. As in this case, it is very rare that it occurs at the end of the digital extremity. The skin may appear normal or occasionally ulcerated, pink, purple, or blue-colored skin [
1]. It is characterized by asymptomatic and sudden growth in size over a long period of time, and this rapid growth is caused by external stimuli such as trauma, electrocautery, or incomplete excision [
5]. MNH has high local recurrence rate (50%), followed by metastasis to lymph nodes, bone, and visceral organs (60%), usually fatal results [
6].
Diagnostic clues for MNH included the large size of the lesions, poor circumscription with dispersed tumor lobules infiltrating tissues at a notable distance from the parent tumor mass, solid sheet-like growth pattern, comedo pattern of tumor necrosis, presence of lymphatic invasion, high nucleocytoplasmic ratios, and frequent mitotic figures [
1]. MNH differentiated histologically from other clear cell tumors of skin, such as squamous cell carcinoma, basal cell carcinoma, and other adnexal carcinomas. Basal cell carcinoma and squamous cell carcinoma differ in ductal formation, showing cuticular differentiation and multilobular infiltrative growth pattern. Differential diagnosis with other malignant adnexal carcinoma is important because of similar cytologic features. The nuclei of malignant sebaceous tumor cells show prominent indentations with distinctive scalloping. Eccrine porocarcinoma is readily distinguished by basaloid proliferation of cells with intra-epidermal nesting and lack of clear cell transformation [
1].
In MRI, both T1- and T2-weighted images show low signal intensity, and malignancy can be predicted by referring to the clarity of the tumor margin and the degree of invasion of surrounding tissues [
7].
Treatment requires extensive surgical resection due to the aggressive nature of the tumor and high local recurrence and metastasis rates. In the advanced stage of SGC, a safety margin of 0.5 to 1.0 cm should be secured with digital amputation [
3]. In 1990, it was first reported that Dhawan treated SGC at the fingertip through Mohs micrographic surgery [
8]. Ten years later, a case in which Chang et al. [
2] treated MNH on the fingertip of the right index finger with clear resection margin was introduced. In the above cases, no focal lymph node or distant metastasis was observed. Therefore, only margin-free resection was used to finish treatment without recurrence and prevent distal amputation [
2]. In our case, bone erosion was observed in the image based on the pathological findings. Thus, it resulted in distal amputation.
According to Robson et al. [
9], high mitotic counts, lymphovascular invasion, and tumor depth greater than 7 mm in SGC have a poor prognosis and require sentinel lymph node biopsy (SLNB). SLNB helps to identify metastasis early. We did not perform SLNB because we did not suspect malignancy at the time of the first operation, and we confirmed that there was no metastasis through PET-CT at the cancer center.
We missed the bone erosion in the preoperative X-ray and MRI, and the small bone erosion was not visually confirmed even during the operation, so we suspected it to be benign and performed the operation.
In conclusion, for a mass that grows slowly, rapidly increases in size, and shows ulcers on the skin, an accurate diagnosis should be made through accurate imaging and histological examination after surgery. Also, if margin-free resection can be performed during the second operation, digital amputation should be avoided, but if tumor margins show evidence of bone invasion, local recurrence and metastasis should be prevented through digital amputation.
Go to :






 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download