Discussion
In this study, we evaluated the effect of prophylactic LVA on the prevention of arm lymphedema in breast cancer patients after ALND. Most patients in our study had high-risk factors for lymphedema, undergoing ALND and adjuvant regional lymph node radiotherapy (76.8%). Most patients underwent adjuvant chemotherapy (78.3%). Although controversial, this risk factor has been independently associated with lymphedema development in some studies [
9-
11]. None of the patients in the treatment group were diagnosed with BCRL (0%). As observed from this result, there is a promising prospect of successful prevention of lymphedema in patients with high-risk factors for developing lymphedema by performing prophylactic LVA; and we argue that this finding presents an opportunity to expand the scope of applying preventive LVA in other patient groups at high risk for lymphedema as well as in breast cancer patients.
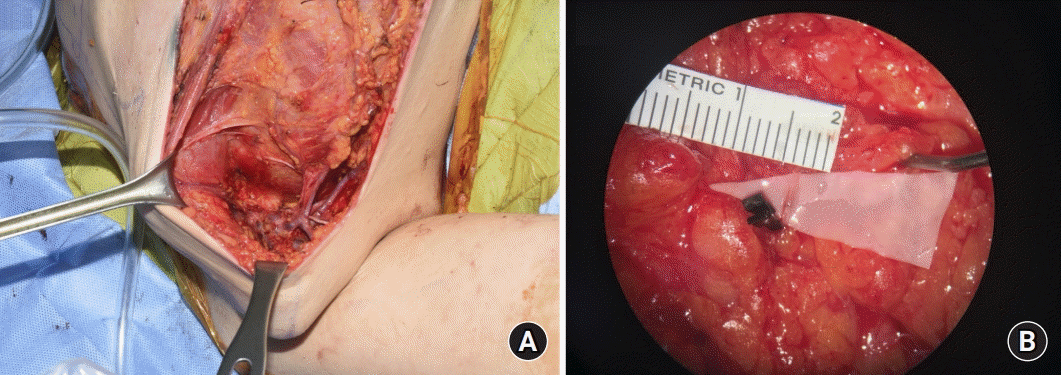
Nevertheless, the wide variability in the prevalence of cancer-related arm lymphedema and the rate of lymphedema are high. Sentinel lymph node biopsy was performed to prevent cancer-related lymphedema, but recent studies have argued that with sentinel lymph node biopsy alone, lymphedema rates cannot be ignored [
12,
13]. Thus, prevention is a key factor in avoiding lymphedema. In this study, the dissection of lymph nodes or lymphatics responding to the gamma-ray detector was performed while performing ALND by injecting technetium-99 radiolabeled colloid into the subcutaneous fat around the breast mass at four positions at 3, 6, 9, and 12 o’clock. The preservation status of the lymphatics or lymph nodes in the arm was not confirmed. If the lymphatic pathway from the arm could be visualized and confirmed using the axillary reverse mapping technique introduced in recent studies [
14,
15] and avoided during lymph node dissection, more arm lymphatic drainage could be preserved. However, as discussed by Boccardo [
16], even when ALND was performed using the axillary reverse mapping technique, it was almost impossible to identify efferent lymphatics that truly preserved the lymphatic flow in the arm, because arm lymphatic drainage combines the common lymphatic pathways draining the breasts. Therefore, since preservation is practically impossible, in this study, prophylactic LVA was performed at the same time as ALND.
Many LVA shunting procedures have been developed. In this study, prophylactic LVA was performed using supermicrosurgical LVA, and none of the 21 patients who underwent surgical prevention developed lymphedema (0%). This rate was lower than that of 4.34% in primary prevention of arm lymphedema using the telescopic LVA technique presented by Boccardo [
16]. However, since this study only presents a follow-up period of 6 months, which is shorter than the follow-up period in Boccardo’s research, it is necessary to perform further evaluation with a longer period for an accurate comparison.
LVA with telescopic technique uses larger veins, which are a major cause of increased intraluminal pressure, which can lead to venous-lymphatic reflux and thrombosis [
17]. Supermicrosurgical LVA aims to overcome this problem by anastomosing lymphatics and venules <0.8 mm in diameter in an intima-to-intima manner, which supposedly has a lower occlusion rate, by minimizing backflow and nonendothelial tissue in the vessel lumen [
18]. In addition, a recent animal study revealed that supermicrosurgical LVA patency rates were higher than those of lymphovenous implantation [
19]. Therefore, although this is a preliminary study, there was no patient who developed lymphedema among those who underwent supermicrosurgical LVA as the primary prevention; thus, it is expected that with a longer follow-up period and higher number of patients, the proposed supermicrosurgical LVA will establish itself as a competitive technique with a lower rate of lymphedema compared to telescopic LVA.
The disadvantage of supermicrosurgical LVA is that the operation time is longer than that of the telescopic LVA technique. In most of the previous studies, the supermicrosurgical LVA technique for lymphedema prevention was performed at heterotopic locations, which are different from those used in lymphadenectomy, and the operation time measured in these studies ranged from approximately 30 minutes to 2 hours [
20,
21]. In this study, LVA was performed in the axillary region where lymphadenectomy was executed to reduce the operation time, which is a weakness of the supermicrosurgical LVA technique; thus, LVA was performed in isotopic rather than in heterotopic locations. In this manner, the time taken for operation was approximately 30 minutes to 1 hour, which resulted in a reduction in the operation time by approximately 1 hour compared to performing LVA at heterotopic locations. Furthermore, there were no significant expenses for prophylactic LVA because it was immediately preceded after ALND and required only 30 to 60 minutes to perform. The microscope, microsurgical and supermicrosurgical tools, and surgeons were already available, and the cost of suture material was negligible.
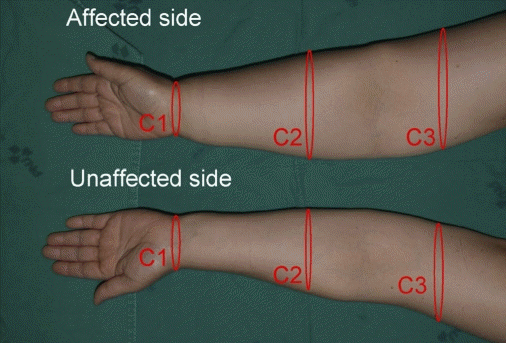
Among the various methods for lymphedema diagnosis, the measurement of arm circumference is the most common technique. Measuring the circumference of the arm is simple and convenient without the need for specific equipment or space, but it requires interobserver reliability that cannot be achieved. However, circumferential measurements have been found to be accurate and reliable when performed by trained staff [
22]. Therefore, in this study, a single trained plastic surgeon performed these measurements in all patients to reduce interobserver variability as much as possible. In addition, ICG lymphography was performed in all patients at 6 months of follow-up, and imaging was done to assess the functions of the lymphatic drainage channels. The presence of DB was confirmed in all patients with differences in bilateral arm circumference of more than 2 cm; no DB was confirmed in patients with a respective difference of <2 cm, and they showed a linear lymphatic pattern.
Swelling that occurred in the arm on the site of oncological surgery in the first year is likely transient lymphedema [
23]. The rate of transient lymphedema is approximately 10.8% to 71.0% [
23-
26]. However, each transient lymphedema study has a different way of measuring and defining the disease, leading to variability in the rate of occurrence [
24].
The majority of postoperative transient lymphedema diagnoses resolved completely within 3 to 6 months of onset [
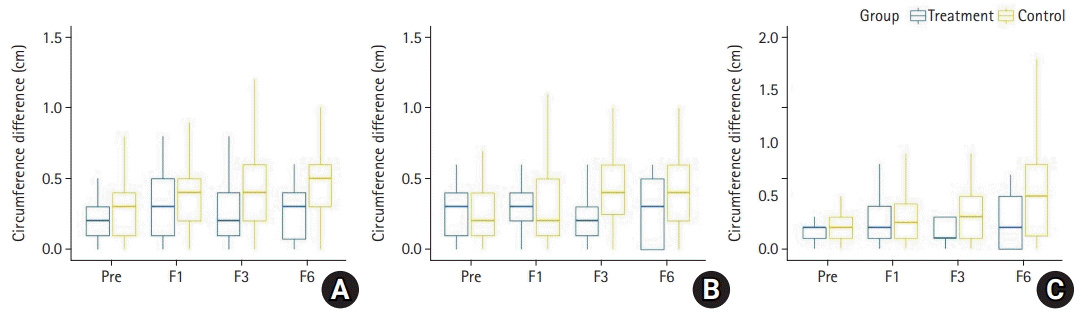
24]. In the present study, the treatment group underwent oncological surgery, followed by prophylactic LVA. The evaluation of lymphedema was performed at 1, 3, and 6 months postoperatively, and the evaluation method included the measurements of the circumference of both arms and ICG lymphography. However, volume analysis or bioimpedance spectroscopy evaluation was not performed. None of the patients in the treatment group had >2 cm difference in the three areas of the arm circumference measured at 1, 3, and 6 months postoperatively; no DB was observed on ICG lymphography in any of the patients at 6 months postoperatively.
While we do not believe that the rate of transient lymphedema is zero in the treatment group, it is thought that the rate of arm swelling can certainly be reduced in the treatment group compared to the control group since primary prophylactic LVA was performed. The rate of transient lymphedema is 0, probably due to the small number of patients in the treatment group, or some patients may have undergone transient lymphedema, but swelling may not have occurred during the clinic visits after the surgery. It could also be that the transient lymphedema had occurred between the visits and resolved before the next visit. Other studies report a lower rate of lymphedema occurrence after the prophylactic lymphedema surgery compared to the typical rate of transient lymphedema [
16,
27,
28]; therefore, it can be thought that the prophylactic lymphedema surgery lowers the rate of lymphedema.
The incidence rate of BCRL ranges from 3% to 65%, and this can be due to the surgical treatment approach, the diagnosis of lymphedema, and the duration of follow-up [
29-
31]. The occurrences of BCRL were 4.4%, 10.1%, 15.2%, 28.6%, 31.2%, and 32.5% at 1, 3, 6, 12, 18, and 24 months after surgery, respectively [
32]. According to recent reports, a primary lymphatic insufficiency state was identified on ICG lymphography, which may explain an increased likelihood of lymphedema after oncological treatment in patients with the preexisting disease compared with their healthy counterparts [
33]. Therefore, it is seen that the pathologic changes in the lymphatic vessels occur before the symptoms of lymphedema in patients who received oncological treatment. While transient lymphedema can occur in both control and treatment groups, the incidence of BCRL was 0% at 6 months postoperatively in the treatment group and 18.8% in the control group. This is similar to the rate of lymphedema reported in other studies and may be considered reliable [
16,
32]. Although the follow-up duration is short, a statistically significant difference in the rate of lymphedema was observed during the study periods, and this is thought to be sufficient to demonstrate the effects of prophylactic lymphedema surgery. Therefore, the fact that there is a difference in the rate of lymphedema clearly indicates that preventive LVA is effective in the prevention of lymphedema. If a patient had a difference in arm circumference measurements of >2 cm at the postoperative outpatient visit or if DB was observed on lymphography, it was determined that the patient developed lymphedema, and physical therapy and compression bandage/stocking were performed in these patients.
In the treatment group, there was a minimal difference in arm circumference measured at 1, 3, and 6 months postoperatively, but the difference in arm circumference in the control group gradually increased at 1, 3, and 6 months postoperatively. However, this difference between the two groups was not statistically significant over time. This study was a preliminary study, and variations in arm circumference were measured during the follow-up period of 6 months postoperatively. It is believed that the difference in arm circumference in the control group would increase with an increase in the length of the follow-up period. Thus, it is likely that the difference in arm circumference between the two groups over time will be statistically significant.
Different follow-up periods were considered when evaluating the effect of prophylactic LVA on BCRL. Some studies argued that a minimum follow-up period of 3 years after initiating breast cancer treatment should be considered to adequately detect patients at risk of developing postoperative lymphedema [
5,
34]. This preliminary study had 1-, 3-, and 6-months follow-up periods, with a mean follow-up period of less than 12 months. This follow-up period is considered relatively short because, in the majority of patients, lymphedema develops approximately during the first year after breast cancer treatment. However, this preliminary study made a significant contribution because it has demonstrated that prophylactic LVA significantly prevented the occurrence of lymphedema after ALND. Therefore, the authors contend that, based on the findings of this study, the effect of prophylactic LVA for the prevention of lymphedema can be clearly demonstrated if the length of the follow-up period is increased to 3 years or longer and the number of patients is sufficiently increased.
In this study, there was a difference in the number of patients between the two groups. The reasons for the greater number of patients in the control group included the patient’s refusal to undergo prophylactic LVA, a change in the surgery schedule, and sudden change in the plan to perform ALND. The sudden change in the plan to perform ALND occurred because the metastasis finding of the axillary lymph node was negative on core needle biopsy at outpatient visiting; however, the metastasis finding of the axillary region was positive on sentinel lymph node biopsy at operation after undergoing mastectomy or lumpectomy. Some of the patients lacked a clear understanding of lymphedema, or they thought it would not happen to them, and there was also a reluctance to undergo preventive surgery. This study demonstrated a positive effect of prophylactic LVA as the primary strategy for BCRL prevention in patients who were referred for a lymph node dissection. Therefore, providing active education to patients who will undergo ALND on lymphedema and providing them with appropriate knowledge on the effectiveness of prophylactic LVA for primary preservation of BCRL, can improve their quality of life by reducing the BCRL rate.
We demonstrated the effects of prophylactic LVA. Unfortunately, patients in the control group did not receive any prophylactic CDT before the diagnosis of lymphedema. Therefore, comparative studies between prophylactic CDT and prophylactic LVA for the prevention of BCRL are required.
There are some limitations to our study. First, although our study has powerful strengths, including multiple measurement methods of lymphedema, it was not designed as a randomized trial. Second, our study was limited by the number of patients in the treatment group for analysis. Third, our follow-up period was 6 months, which was a short time to evaluate the exact incidence of lymphedema.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download