1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021; 143:e254–e743. PMID:
33501848.
2. He L, Zhou B. Cardiomyocyte proliferation: remove brakes and push accelerators. Cell Res. 2017; 27:959–960. PMID:
28707671.

3. Ludhwani D, Abraham J, Kanmanthareddy A. StatPearls. Heart transplantation rejection [Internet]. Treasure Island (FL): StatPearls Publishing;2021. 03. 12. cited 2021 July 22. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK537057/.
4. Bar A, Cohen S. Inducing endogenous cardiac regeneration: can biomaterials connect the dots? Front Bioeng Biotechnol. 2020; 8:126. PMID:
32175315.

5. Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007; 21:1345–1357. PMID:
17284483.

6. Uto K, Tsui JH, DeForest CA, Kim DH. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog Polym Sci. 2017; 65:53–82. PMID:
28522885.

7. Williams MA, Mair DB, Lee W, Lee E, Kim DH. Engineering three-dimensional vascularized cardiac tissues. Tissue Eng Part B Rev. 2021; ten.teb.2020.0343.

8. Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012; 44:2060–2064. PMID:
22903023.

9. Kwon S, Shin S, Do M, et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J Control Release. 2021; 330:15–30. PMID:
33278480.

10. Yadid M, Lind JU, Ardoña HA, et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci Transl Med. 2020; 12:eaax8005. PMID:
33055246.

11. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200:373–383. PMID:
23420871.

12. Chun C, Smith AS, Kim H, et al. Astrocyte-derived extracellular vesicles enhance the survival and electrophysiological function of human cortical neurons in vitro. Biomaterials. 2021; 271:120700. PMID:
33631652.

13. Liu B, Lee BW, Nakanishi K, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018; 2:293–303. PMID:
30271672.

14. Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012; 111:312–321. PMID:
22581926.

15. Beltrami C, Besnier M, Shantikumar S, et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther. 2017; 25:679–693. PMID:
28159509.

16. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst). 2010; 4:214–222.

17. Antes TJ, Middleton RC, Luther KM, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology. 2018; 16:61. PMID:
30165851.

18. Wang X, Chen Y, Zhao Z, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018; 7:e008737. PMID:
30371236.

19. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017; 38:201–211. PMID:
28158410.

20. Gao L, Wang L, Wei Y, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020; 12:eaay1318. PMID:
32938792.

21. Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017; 44:2105–2116. PMID:
29241208.

22. Hirai K, Ousaka D, Fukushima Y, et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med. 2020; 12:eabb3336. PMID:
33298561.

23. Hamada T, Dubois JL, Bellamy V, et al. In vitro controlled release of extracellular vesicles for cardiac repair from poly(glycerol sebacate) acrylate-based polymers. Acta Biomater. 2020; 115:92–103. PMID:
32814141.

24. Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005; 97:8–15. PMID:
16002755.

25. Huang K, Ozpinar EW, Su T, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020; 12:9683.

26. Qian Z, Sharma D, Jia W, Radke D, Kamp T, Zhao F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics. 2019; 9:2143–2157. PMID:
31149034.

27. Shah M, Kc P, Zhang G. In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch. ACS Appl Mater Interfaces. 2019; 11:23893–23900. PMID:
31188555.

28. Chen J, Zhan Y, Wang Y, et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018; 80:154–168. PMID:
30218777.

29. Pok S, Benavides OM, Hallal P, Jacot JG. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng Part A. 2014; 20:1877–1887. PMID:
24433519.

30. Kapnisi M, Mansfield C, Marijon C, et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv Funct Mater. 2018; 28:1800618. PMID:
29875619.

31. Hosoyama K, Ahumada M, McTiernan CD, et al. Nanoengineered electroconductive collagen-based cardiac patch for infarcted myocardium repair. ACS Appl Mater Interfaces. 2018; 10:44668–44677. PMID:
30508481.

32. Lakshmanan R, Kumaraswamy P, Krishnan UM, Sethuraman S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials. 2016; 97:176–195. PMID:
27177129.

33. O’Neill HS, O’Sullivan J, Porteous N, et al. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J Tissue Eng Regen Med. 2018; 12:e384–e394. PMID:
27943590.

34. Serpooshan V, Zhao M, Metzler SA, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013; 34:9048–9055. PMID:
23992980.

35. Cui H, Liu C, Esworthy T, et al. 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci Adv. 2020; 6:eabb5067. PMID:
32637623.

36. Gao L, Kupfer ME, Jung JP, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. 2017; 120:1318–1325. PMID:
28069694.

37. Wang QL, Wang HJ, Li ZH, Wang YL, Wu XP, Tan YZ. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J Cell Mol Med. 2017; 21:1751–1766. PMID:
28244640.

38. Abbasgholizadeh R, Islas JF, Navran S, Potaman VN, Schwartz RJ, Birla RK. A highly conductive 3D cardiac patch fabricated using cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells. Cardiovasc Eng Technol. 2020; 11:205–218. PMID:
31916039.

39. Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014; 15:750–761. PMID:
25479750.

40. Mehrotra S, de Melo BA, Hirano M, et al. Nonmulberry silk based ink for fabricating mechanically robust cardiac patches and endothelialized myocardium-on-a-chip application. Adv Funct Mater. 2020; 30:1907436. PMID:
33071707.

41. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005; 23:47–55. PMID:
15637621.

42. Chen WL, Kan CD. Using cell-seeded electrospun patch for myocardial injury: in-vitro and in rat model. Annu Int Conf IEEE Eng Med Biol Soc. 2018; 2018:5338–5341. PMID:
30441542.

43. Rai R, Tallawi M, Barbani N, et al. Biomimetic poly(glycerol sebacate) (PGS) membranes for cardiac patch application. Mater Sci Eng C. 2013; 33:3677–3687.

44. Ravichandran R, Venugopal JR, Mukherjee S, Sundarrajan S, Ramakrishna S. Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Eng Part A. 2015; 21:1288–1298. PMID:
25559869.

45. Cristallini C, Vaccari G, Barbani N, et al. Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: a feasibility study. J Tissue Eng Regen Med. 2019; 13:1253–1264. PMID:
31050859.

46. Bahrami S, Solouk A, Mirzadeh H, Seifalian AM. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos, Part B Eng. 2019; 168:421–431.

47. Chung HJ, Kim JT, Kim HJ, et al. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release. 2015; 205:218–230. PMID:
25681051.

48. Spadaccio C, Nappi F, De Marco F, et al. Implantation of a poly-L-lactide GCSF-functionalized scaffold in a model of chronic myocardial infarction. J Cardiovasc Transl Res. 2017; 10:47–65. PMID:
28116550.

49. Pushp P, Bhaskar R, Kelkar S, Sharma N, Pathak D, Gupta MK. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol Bioeng. 2021; 118:2312–2325. PMID:
33675237.

50. Su T, Huang K, Daniele MA, et al. Cardiac stem cell patch integrated with microengineered blood vessels promotes cardiomyocyte proliferation and neovascularization after acute myocardial infarction. ACS Appl Mater Interfaces. 2018; 10:33088–33096. PMID:
30188113.

51. Tang J, Wang J, Huang K, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018; 4:eaat9365. PMID:
30498778.

52. Park BW, Jung SH, Das S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020; 6:eaay6994. PMID:
32284967.

53. Wang Q, Yang H, Bai A, et al. Functional engineered human cardiac patches prepared from nature's platform improve heart function after acute myocardial infarction. Biomaterials. 2016; 105:52–65. PMID:
27509303.

54. Weinberger F, Breckwoldt K, Pecha S, et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016; 8:363ra148.

55. Gao L, Gregorich ZR, Zhu W, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018; 137:1712–1730. PMID:
29233823.

56. Schaefer JA, Guzman PA, Riemenschneider SB, Kamp TJ, Tranquillo RT. A cardiac patch from aligned microvessel and cardiomyocyte patches. J Tissue Eng Regen Med. 2018; 12:546–556. PMID:
28875579.

57. Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh). 2019; 6:1900344. PMID:
31179230.

58. Kim DH, Lipke EA, Kim P, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010; 107:565–570. PMID:
20018748.

59. Kshitiz AJ, Afzal J, Kim SY, Kim DH. A nanotopography approach for studying the structure-function relationships of cells and tissues. Cell Adhes Migr. 2015; 9:300–307.

60. Mengsteab PY, Uto K, Smith AS, et al. Spatiotemporal control of cardiac anisotropy using dynamic nanotopographic cues. Biomaterials. 2016; 86:1–10. PMID:
26874887.

61. Carson D, Hnilova M, Yang X, et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces. 2016; 8:21923–21932. PMID:
26866596.

62. Tsui JH, Janebodin K, Ieronimakis N, et al. Harnessing sphingosine-1 phosphate signaling and nanotopographical cuesto regulate skeletal muscle maturation and vascularization. ACS Nano. 2017; 11:11954–11968. PMID:
29156133.
63. Kim DH, Kshitiz , Smith RR, et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol. 2012; 4:1019–1033.

64. Lin YD, Ko MC, Wu ST, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci. 2014; 2:567–580. PMID:
26827729.

65. Macadangdang J, Lee HJ, Carson D, et al. Capillary force lithography for cardiac tissue engineering. J Vis Exp. 2014; e50039.

66. Kim P, Yuan A, Nam KH, Jiao A, Kim DH. Fabrication of poly(ethylene glycol): gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication. 2014; 6:024112. PMID:
24717683.

67. Uto K, Aoyagi T, Kim DH, Ebara M. Free-standing nanopatterned poly(ε-caprolactone) thin films as a multifunctional scaffold. IEEE Trans NanoTechnol. 2018; 17:389–392.

68. Penland N, Choi E, Perla M, Park J, Kim DH. Facile fabrication of tissue-engineered constructs using nanopatterned cell sheets and magnetic levitation. Nanotechnology. 2017; 28:075103. PMID:
28028248.

69. Jiao A, Trosper NE, Yang HS, et al. Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano. 2014; 8:4430–4439. PMID:
24628277.

70. Williams NP, Rhodehamel M, Yan C, et al. Engineering anisotropic 3D tubular tissues with flexible thermoresponsive nanofabricated substrates. Biomaterials. 2020; 240:119856. PMID:
32105818.

71. Malki M, Fleischer S, Shapira A, Dvir T. Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 2018; 18:4069–4073. PMID:
29406721.

72. Smith AS, Yoo H, Yi H, et al. Micro- and nano-patterned conductive graphene-PEG hybrid scaffolds for cardiac tissue engineering. Chem Commun (Camb). 2017; 53:7412–7415. PMID:
28634611.

73. Tsui JH, Ostrovsky-Snider NA, Yama DMP, et al. Conductive silk-polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. J Mater Chem B. 2018; 6:7185–7196. PMID:
31448124.

74. Choi JS, Smith AS, Williams NP, et al. Nanopatterned Nafion microelectrode arrays for in vitro cardiac electrophysiology. Adv Funct Mater. 2020; 30:1910660. PMID:
33244297.

75. Smith AS, Choi E, Gray K, et al. NanoMEA: a tool for high-throughput, electrophysiological phenotyping of patterned excitable cells. Nano Lett. 2020; 20:1561–1570. PMID:
31845810.
76. Choi JS, Lee HJ, Rajaraman S, Kim DH. Recent advances in three-dimensional microelectrode array technologies for in vitro and in vivo cardiac and neuronal interfaces. Biosens Bioelectron. 2021; 171:112687. PMID:
33059168.

77. Peña B, Laughter M, Jett S, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018; 18:e1800079. PMID:
29733514.

78. Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014; 5:3935. PMID:
24887553.

79. Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymers (Basel). 2011; 3:740–761.

80. Shin YJ, Shafranek RT, Tsui JH, Walcott J, Nelson A, Kim DH. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater. 2021; 119:75–88. PMID:
33166713.

81. Mandrycky C, Wang Z, Kim K, Kim DH 3rd. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016; 34:422–434. PMID:
26724184.

82. Das S, Jang J. 3D bioprinting and decellularized ECM-based biomaterials for in vitro CV tissue engineering. J 3D Printing Med. 2018; 2:69–87.
83. Anker SD, Coats AJ, Cristian G, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J. 2015; 36:2297–2309. PMID:
26082085.

84. Lee LC, Wall ST, Klepach D, et al. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int J Cardiol. 2013; 168:2022–2028. PMID:
23394895.

85. Rao SV, Zeymer U, Douglas PS, et al. Bioabsorbable intracoronary matrix for prevention of ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2016; 68:715–723. PMID:
27515331.

86. Traverse JH, Henry TD, Dib N, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl Sci. 2019; 4:659–669. PMID:
31709316.

87. Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011; 4:528–542. PMID:
21710332.

88. Wang H, Zhou J, Liu Z, Wang C. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010; 14:1044–1055. PMID:
20193036.

89. Contessotto P, Orbanić D, Da Costa M, et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci Transl Med. 2021; 13:eaaz5380. PMID:
33597263.

90. Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg. 2008; 85:901–908. PMID:
18291168.

91. Hoeeg C, Dolatshahi-Pirouz A, Follin B. Injectable hydrogels for improving cardiac cell therapy-in vivo evidence and translational challenges. Gels. 2021; 7:7. PMID:
33499287.

92. Tsui JH, Leonard A, Camp ND, et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials. 2021; 272:120764. PMID:
33798964.

93. Liao X, Yang X, Deng H, et al. Injectable hydrogel-based nanocomposites for cardiovascular diseases. Front Bioeng Biotechnol. 2020; 8:251. PMID:
32296694.

94. Deng C, Zhang P, Vulesevic B, et al. A collagen–chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng Part A. 2010; 16:3099–3109. PMID:
20586613.

95. Song Y, Zhang C, Zhang J, et al. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016; 41:210–223. PMID:
27262742.

96. Wang H, Liu Z, Li D, et al. Injectable biodegradable hydrogels for embryonic stem cell transplantation: improved cardiac remodelling and function of myocardial infarction. J Cell Mol Med. 2012; 16:1310–1320. PMID:
21838774.

97. Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009; 54:1014–1023. PMID:
19729119.

98. Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016; 118:330–343. PMID:
26838317.
99. Hasan A, Khattab A, Islam MA, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinh). 2015; 2:1500122. PMID:
27668147.

100. Chaterji S, Ahn EH, Kim DH. CRISPR genome engineering for human pluripotent stem cell research. Theranostics. 2017; 7:4445–4469. PMID:
29158838.

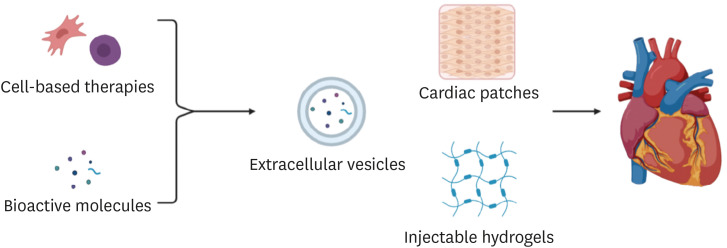
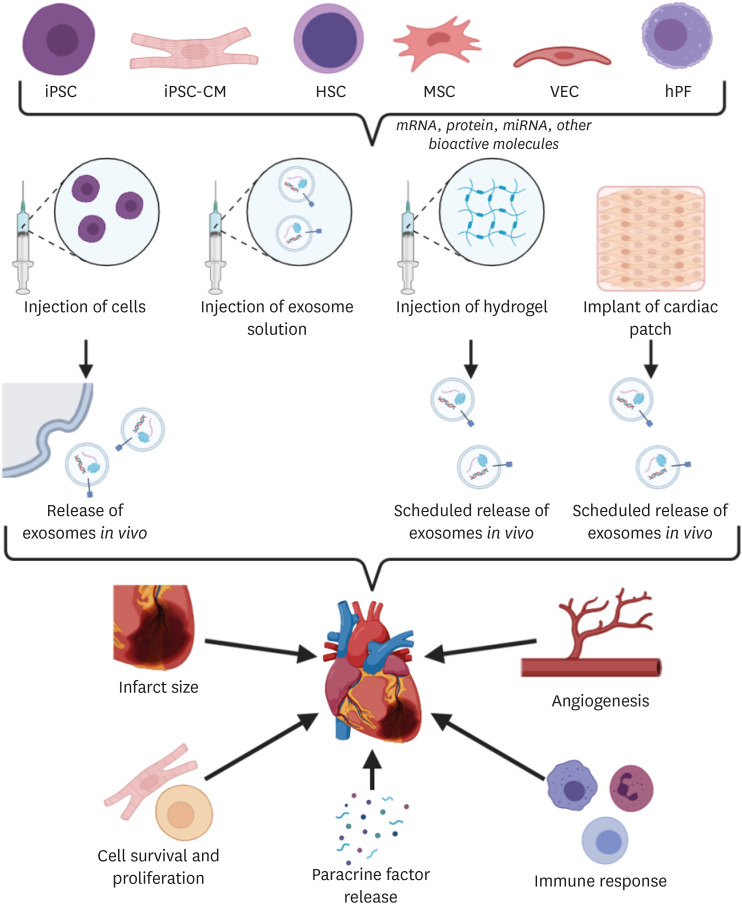
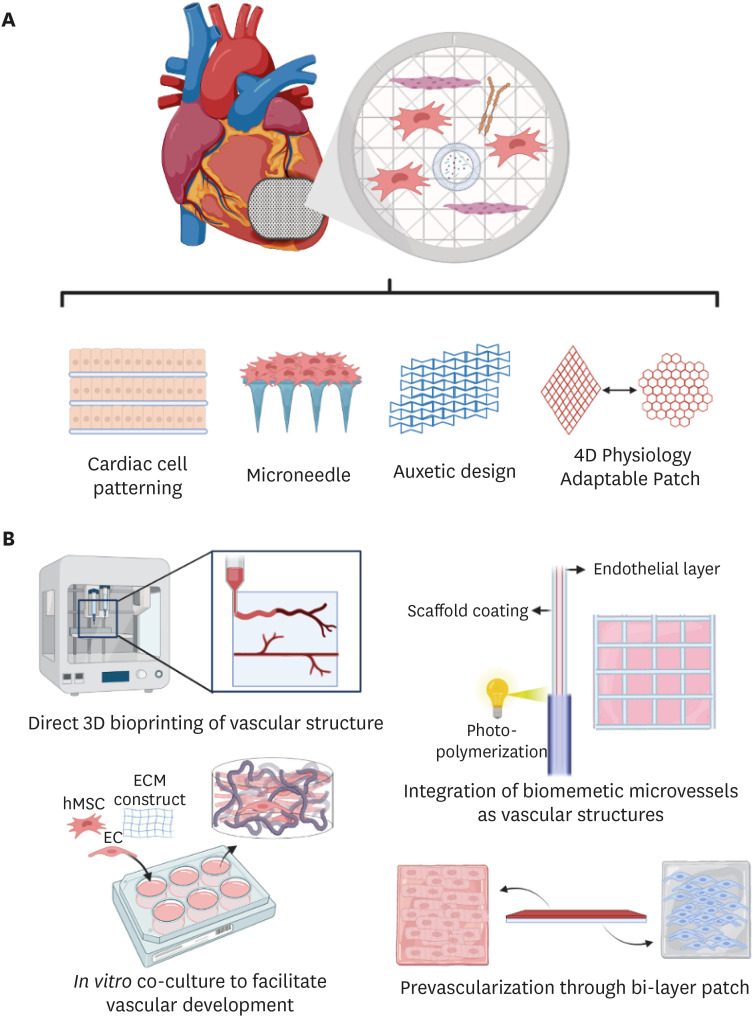

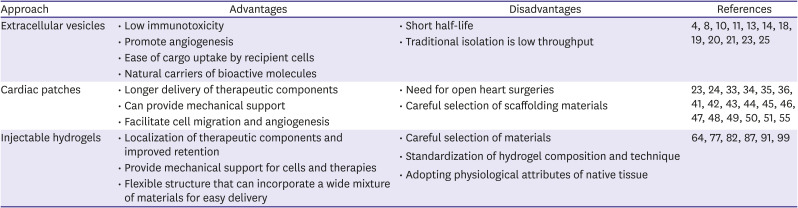




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download