INTRODUCTION
Atrial septal defect (ASD) is among the most commonly reported congenital heart diseases (CHDs), accounting for about 10% of all CHDs in children,
1) with secundum ASD comprising approximately 80% of the ASDs.
2)
Some patients with a large ASD remain asymptomatic until adulthood
2); however, if left untreated, symptoms such as dyspnea, arrhythmia, reduced functional capacity, right heart failure, respiratory infections, and stroke may present through right ventricle (RV) volume overload and pulmonary overflow.
2)3)
Although both transcatheter and surgical closures yield excellent short and long-term ASD treatment outcomes,
4) several studies have reported the superiority of transcatheter closure to surgery in terms of hospitalization duration, costs, and rates of treatment-related complications and infections.
4)5)
As a part of ASD treatment, diuretics are occasionally administered to reduce the rate of RV volume overload for the control of heart failure-related symptoms
6); however, the effect of diuretics on ASD size change remains unclear. This study aimed to evaluate the efficacy of diuretics in ASD size reduction by comparing patients who received and did not receive diuretic treatment, and their outcomes.
METHODS
Ethical statement
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2003-191-1112) and the need for consent was waived owing to the retrospective study design.
Patient characteristics
We retrospectively reviewed the medical records of pediatric patients diagnosed with a large secundum ASD (size ≥10 mm), as observed on echocardiography, at the Seoul National University Children's Hospital between 2005 and 2019. Patients with other concomitant CHDs, such as a ventricular septal defect or patent ductus arteriosus, and those aged ≥10 years were excluded. Patients were divided into two groups based on their diuretic reception status. Patients who received diuretics and were followed-up for a sufficient duration (approximately 2 years) were assigned to the diuretic group. If parents wanted device closure to be performed after the patient had grown a little more while not requiring immediate ASD closure, diuretics were given for the purpose of RV volume unloading. Age-matched patients who were not treated with diuretics for a sufficient duration during study period were assigned to the non-diuretic group. Data regarding the patients' gestational age, weight, height, genetic defect, clinical symptoms, and the use of diuretics and treatment, including transcatheter and surgical closures, were collected.
Echocardiographic measurement of atrial septal defect
Of the ASD images recorded from various angles, the largest measured ASD size was selected, and for data consistency, the ASD size was re-evaluated from the ASD images measured at the same view wherever possible. Since the patient's somatic growth occurred during the administration of the diuretics, the indexed ASD diameter was also evaluated to compare the ASD size over the patient's body surface area (BSA) for the correction of body size differences. From the available images, aortic, superior vena cava (SVC), inferior vena cava (IVC), posterior, and mitral rim measurements were performed. Rim deficiency was defined as a rim size <5 mm. The presence of a floppy interatrial septum, multiple ASDs, and other heart anomalies were evaluated. All patients' echocardiography findings, as obtained during follow-up, were investigated for serial changes in the ASD size. To decrease the degree of inter-observer variability, the ASD size was remeasured by a cardiologist who was blinded to the patients' information, including their diuretic administration status; these measurements were averaged with the previously recorded ASD size.
Outcome measurements
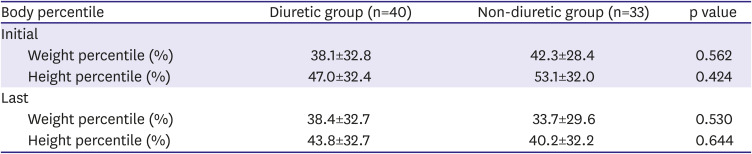
The primary outcome was the effect of diuretics on ASD size reduction and treatment outcomes. The secondary outcome was the identification of the risk factors associated with a lack of ASD size reduction and those related to the need for surgical closure despite diuretic use. Owing to concerns pertaining to growth retardation related to diuretic administration, the percentiles of the patients' height and body weight were evaluated before initiating diuretics and after the reception of follow-up echocardiography. To determine the sex and age-specific percentiles for height and body weight, Korea Centers for Disease Control and Prevention data were used.
7)
Statistical analysis
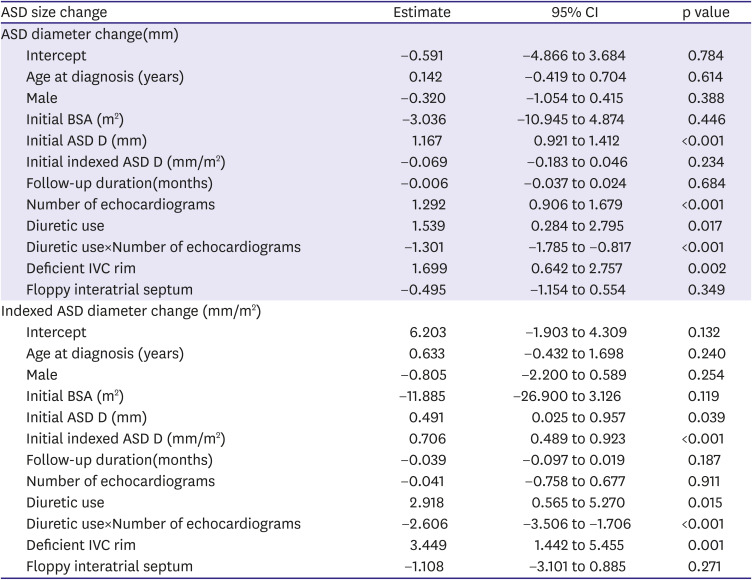
Statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation or median (interquartile range: 25th to 75th percentile) and categorical variables as numbers and percentages. Continuous variables were compared using either Student's t-test or Mann–Whitney U tests and categorical variables by employing χ2 or Fisher's exact tests. Linear mixed model analysis was performed for the identification of meaningful factors associated with ASD diameter and indexed ASD diameter change. A p value of <0.05 was considered statistically significant.
DISCUSSION
In this study, diuretic administration seemed to be associated with changes in ASD size in pediatric patients with a large secundum ASD. In addition, it may be associated with reducing the need for early surgical ASD closure and increasing the rate of transcatheter closure. To the best of our knowledge, no studies have focused on effect of diuretics on ASD size changes until now.
The success rate of transcatheter ASD closure is approximately 95%, with the feasibility and safety of the technique being previously established.
9)10) The prevalence rate of complications is favorable, as it is 1% in the case of transcatheter ASD closure.
11)12)13) Accordingly, transcatheter secundum ASD closure is preferred over surgery and is being attempted at a younger age;
2)14)15)16) however, recent studies reported both lower periprocedural success rates and an increased risk of periprocedural and long-term complications with the use of transcatheter closure either when body weight is ≤15 kg or in the presence of a large secundum ASD (≥20 mm/m
2).
11)17)18) Therefore, for improved procedural success rates and reduced complication rates, it is reasonable to delay the procedure until the weight is approximately 15 kg.
In general, when the ASD diameter is ≥10 mm, it is known to cause a significant L-R shunt, resulting in RV volume overload.
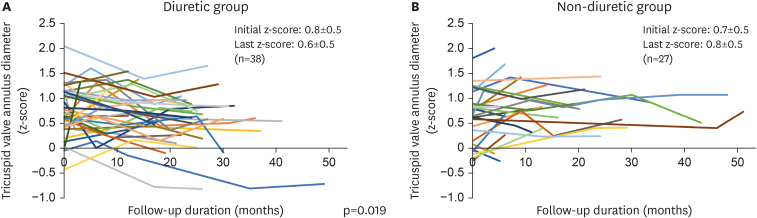
2) We used diuretics for RV volume unloading and expected to use a smaller sized device later through ASD size reduction. We hypothesized that the use of diuretics reduces preload and cause RA and RV volume unloading, thereby reducing the overall RA and RV dimensions and ASD diameter, thus making it possible to use a smaller device. In reality, as shown in
Figure 3, the TV annulus z-score showed a significant difference over time between two groups according to the use of diuretics.
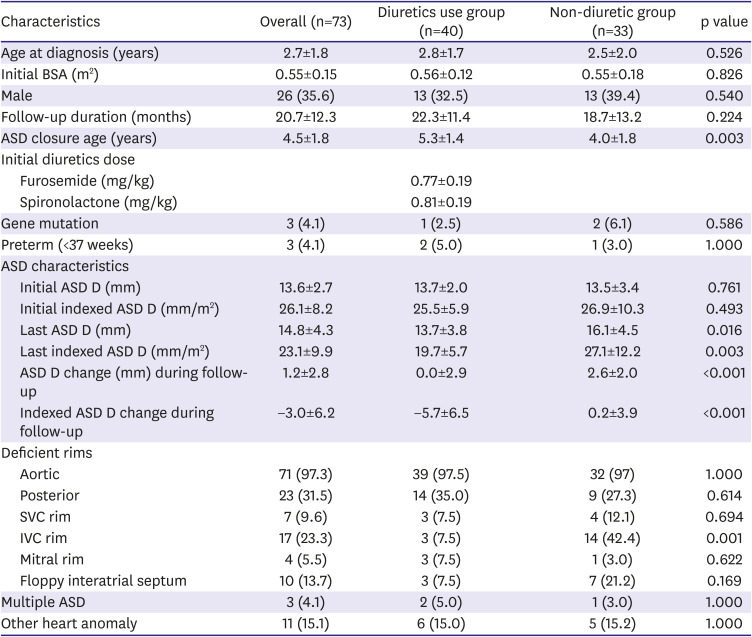
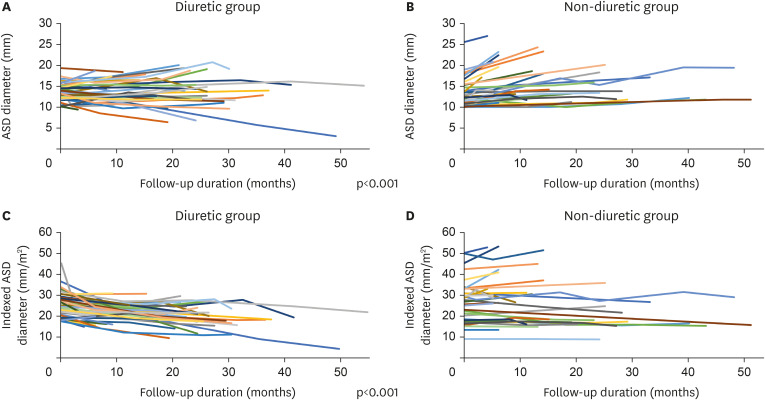
In our study, the ASD size was significantly reduced in the diuretic group compared with the non-diuretic group, and even two patients in the diuretic group showed an ASD size decrease to a level that rendered treatment unnecessary.
19)
Diuretics are used to reduce preload in some patients with CHD.
20) Although there are some concerns about hearing loss associated with the use of high doses of furosemide, recent studies have reported that furosemide and hearing loss are not significantly associated.
21)22)23)24) There is still no clear evidence for complications from long-term use of oral diuretics in children and may be controversial. While concerns might exist regarding the long-term use of diuretics, there were no complications, including those regarding growth retardation at the diuretic dose within 1 mg/kg/day that we used.
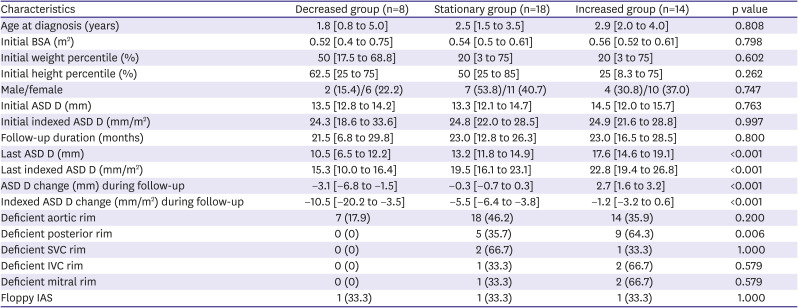
We investigated the diuretic group by dividing it into three subgroups according to the response to diuretics (
Table 3). In the three groups, clinical features did not show any significant difference, and only posterior rim deficiency showed a significant difference and did not have a chance of ASD diameter decrease. The characteristics of the three subgroups according to the ASD size change in the non-diuretic group are presented in
Supplementary Table 1. We found that the ASD size was increased in all patients with posterior rim deficiency in the non-diuretic group. Moreover, all patients with IVC rim deficiency showed an increased ASD diameter in the non-diuretic group. In this study, ASD size reduction was not found in patients with posterior rim deficiency, even among those who received diuretics. Therefore, for patients with posterior rim deficiency, the use of diuretics is likely to be partially effective.
In addition, in our patient group, the number of patients with IVC rim deficiency was significantly higher in the non-diuretic group (7.5 vs. 42.4%, p=0.01), and this difference in distribution of rim deficiency between two groups may have an effect on the difference in treatment trends. So, we investigated whether the distribution of treatment methods was different in the diuretic group and the non-diuretic group even within each subgroup with rim deficiency (
Supplementary Table 2). Since the number of patients in each subgroup is small, there is a limit for analysis, however, when looking at patients with posterior rim deficiency, there was a significant difference in treatment distribution between the diuretic and the non-diuretic group (p=0.046). In addition, while all patients with IVC rim deficiency in the non-diuretic group underwent surgery, one patient in the diuretic group was able to undergo successful device closure, although the number of patients was limited to evaluate it. However, there is still no clear explanation for the relationship between rim deficiency and diuretic effect. Nonetheless, as shown in our data, regardless of the diuretic use, there seemed to be a little possibility of ASD size reduction in case of rim deficiency other than the aortic rim.
Notably, in this study, the mean ASD diameter remained unchanged while the indexed ASD diameter in the diuretic group decreased significantly compared to that in the non-diuretic group. Therefore, in the non-diuretic group, the ASD size increased in accordance with the increase of body growth; However, the use of diuretics seemed to be associated with preventing an increase in the ASD size according to body growth.
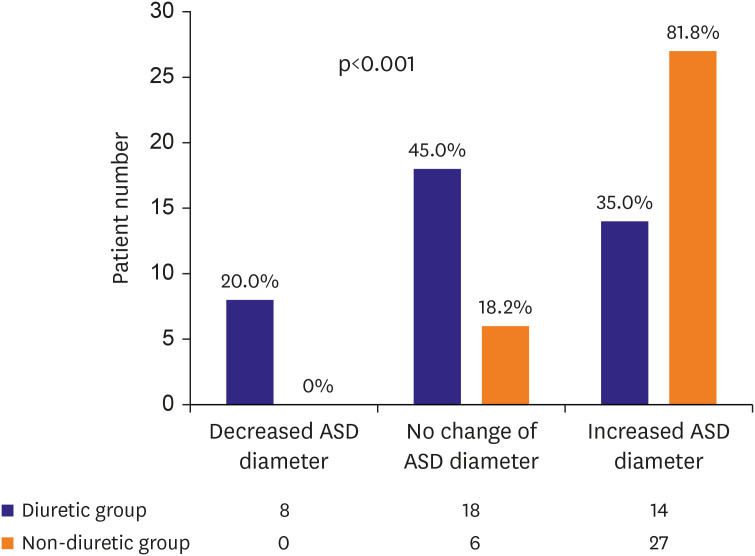
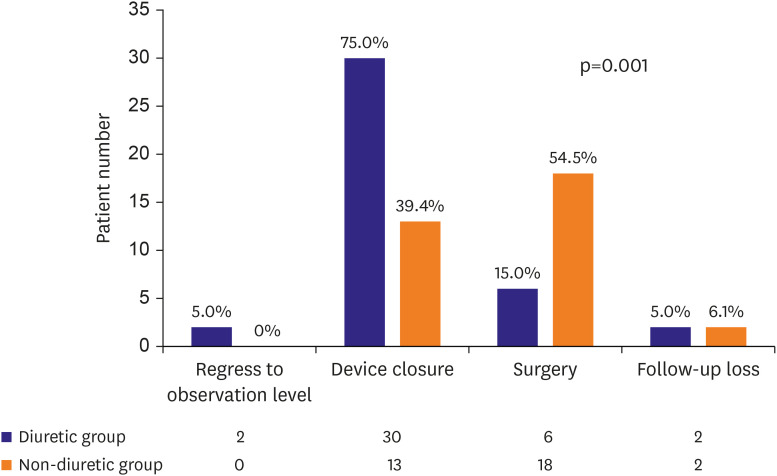
While the effect of diuretics on reducing the ASD size and the resulting outcomes may vary across patients, our data showed that the patients in the diuretic and non-diuretic groups showed significantly different treatment distribution patterns (p=0.001,
Figure 4). The patients with diuretics underwent more non-invasive treatments such as observation or device closure than surgical closure. In addition, even within the diuretic group, the distribution of treatment methods differed depending on whether the patients responded to diuretics (p=0.016,
Supplementary Figure 1). In the group with a decreased ASD size following the use of diuretics, none of the patients underwent surgery. However, 5.6% and 35.7% of the patients in the stationary and the increased groups, respectively, underwent surgery. A patient's response to diuretics positively correlated with the probability of transcatheter device closure than surgical closure.
We investigated patients who underwent surgery in both groups (
Supplementary Table 3). If the ASD size was relatively large and had several rim deficiencies including posterior rim deficiency at the same time, surgical closure was performed more frequently. Though device closure could have been performed in some cases, the surgery was performed according to the preference of the physician or the request of the patients with the large ASDs with several rim deficiencies.
Interestingly, the weight and height growth rates over time did not show any significant difference in the two groups. Of course, it is premature to conclude that the growth rate was not retarded in the diuretic group only from our data, but at least the likelihood of developing specific side effects from diuretic use does not appear to be alarmingly high.
Our study has several limitations. Because of its retrospective design, the potential presence of various biases including selection bias cannot be completely excluded. We included patients with a follow-up period of approximately 6 months or longer, excluding those who were treated immediately after diagnosis, and the follow-up period was similarly matched in both groups. However, it cannot be completely excluded that the consideration of the timing of treatment may have influenced the decision to take diuretics. Additionally, since the total number of patients and the number of patients in each subgroup were small, limitations exist regarding the evaluation of the effect of diuretics, which necessitates the need of randomized controlled trial to get more concrete evidence of the effect of diuretics on ASD size reduction; however, we observed statistically significant differences in the degree of ASD size reduction in diuretic compared to the non-diuretic group.
In pediatric patients with a large secundum ASD (diameter ≥10 mm), diuretic administration seems to be associated with changes in ASD size. The patients receiving diuretics may have a lower possibility to undergo surgical closure. The diuretics administration may be associated with the use of smaller ASD devices for transcatheter treatment through ASD size reduction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download