INTRODUCTION
METHODS
Hospital setting
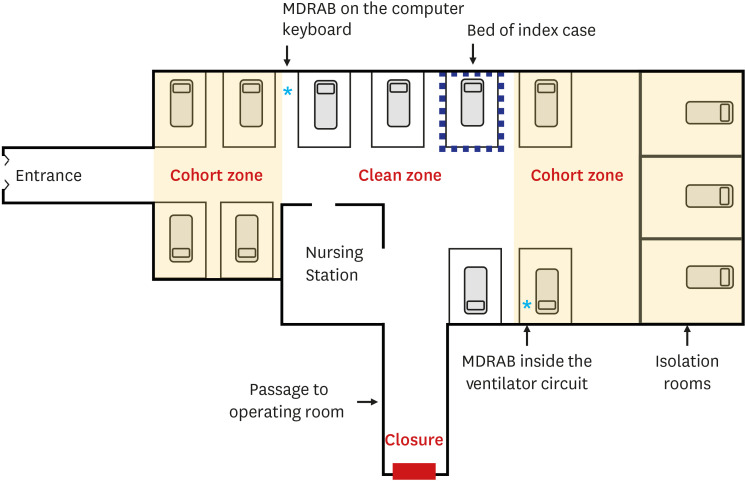 | Fig. 1Schematic map of the pediatric intensive care unit and the implementation of a comprehensive infection-control strategy. MDRAB-colonized patients were isolated in isolation rooms, or in the cohort zone if isolation rooms were unavailable. One entrance was closed. The asterisks represent the sites where MDRAB was detected.MDRAB = multidrug-resistance A. baumannii.
|
Table 1
Summary of the infection-control strategy for MDRAB outbreaks and comparisons with infection-control policies for MDRAB in our hospital
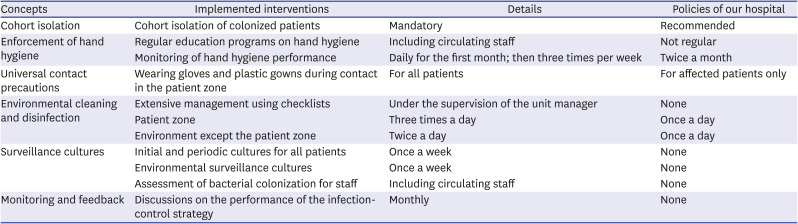
Definitions
Index case and bacterial spread
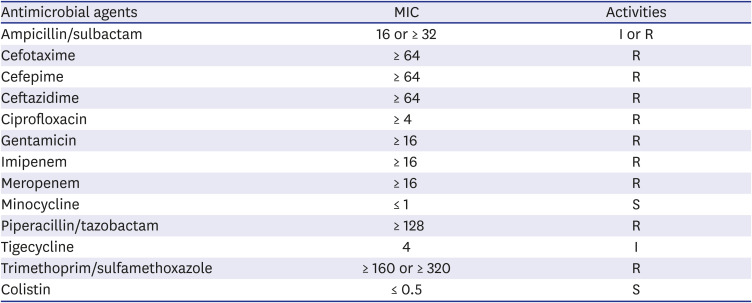
Table 2
In vitro activities of antimicrobial agents against A. baumannii isolated from the index case
Study period and patients
Interventions
1) MDRAB-colonized patients were isolated in isolation rooms. A clean zone was designated, and in the absence of isolation rooms, patients were quarantined in areas other than the clean zone. Patient isolation ended only when three consecutive surveillance bacterial culture tests conducted on alternate days yielded MDRAB-negative results. Medical equipment was used exclusively for one patient if feasible, and shared equipment, such as echocardiogram and electrocardiogram machines, were thoroughly disinfected before and after use. To prevent cross-contact, nurses were separately designated to provide care for either the MDRAB-colonized or non-colonized patients.
2) A pediatric infectious disease specialist and nurses of the Infection Prevention and Control Department imparted regular education programs on hand hygiene for healthcare workers at the PICU and operation rooms; healthcare workers included monthly rotation staff, radiographers, and rehabilitation therapists. The Infection Prevention and Control Department monitored the hand hygiene performance of healthcare workers during work and provided immediate feedback daily for the first month, then three times a week. In addition, aseptic techniques were reinforced for all invasive procedures performed at the PICU and operating room.
3) Regardless of the MDRAB colonization status, universal contact precautions were applied by wearing gloves and plastic gowns during contact with all PICU patients. Contact precautions should be ensured when entering the patient zone, which includes both a patient and his or her surroundings. Healthcare workers received monitoring and feedback from the Infection Prevention and Control Department on appropriate contact precautions, including the donning and removal of personal protective equipment. For patients receiving mechanical ventilation, only a closed suction system was used, and the disconnection of the ventilator circuit was minimized.
4) Environmental management checklists were created to ensure the cleaning and disinfection of the environment. In accordance with the checklist, nursing and cleaning staff thoroughly recorded the site and time of cleaning and disinfection under the supervision of the unit manager. Nursing staff cleaned the environment of the patient zone and the medical equipment during their duty hours three times a day, and the cleaning staff cleaned the walls surrounding patients, computer supplies, tables, washbasins, and doors, twice a day. Computer keyboards were covered with fresh plastic wrap every day after cleaning and wiped with alcohol every hour. Alcohol and various dilutions and concentrations of hypochlorous acid-based disinfectants were used for their intended use in medical equipment and the environment.
5) The initial surveillance culture test was mandated for all patients admitted to the PICU, and periodic cultures were conducted once a week. Culture specimens were collected from tracheal aspirates from intubated or tracheostomized patients and nasopharyngeal swabs from non-intubated patients. Surveillance culture tests for the PICU environment and operating room, including mechanical ventilators, ventilator circuits, suctioning equipment, bedrails, infusion pumps, medication carts, washbasins, and cardiovascular bypass equipment, were performed initially and then followed-up weekly. Nasopharyngeal swab cultures of all related medical staff of the PICU and operating room were performed to assess MDRAB colonization. A specific laboratory code for culture detection of MDRAB was developed to accelerate the detection process and reduce the workload of laboratory technicians.
6) Monthly meetings were held to discuss the performance of the control strategy, results of hand hygiene monitoring, and incidence rate of MDRAB colonization and infection.
Microbiology
Statistical analysis
Ethics statement
RESULTS
Incidence and surveillance
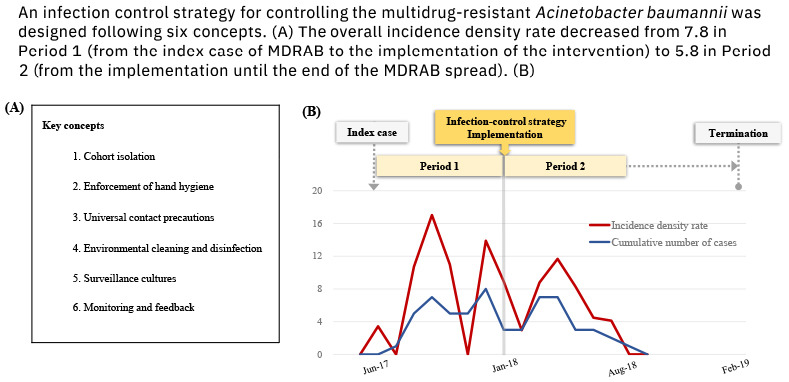
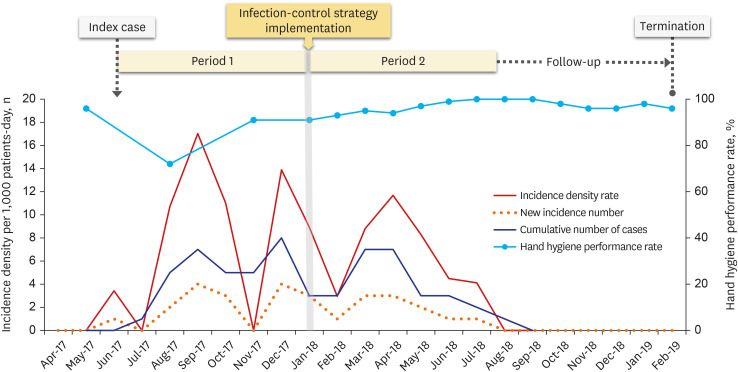 | Fig. 2Monthly incidence of the MDRAB colonization and hand hygiene performance rate during MDRAB outbreak. A comprehensive infection-control strategy was implemented in January 2018. The overall incidence per 1,000 patient days decreased to 5.8 in period 2 from 7.8 in period 1. The last new colonization occurred in July 2018, and the last MDRAB colonization patient was discharged in August 2018. The MDRAB outbreak was declared terminated after the 6-month follow-up following period 2.MDRAB = multidrug-resistance A. baumannii.
|
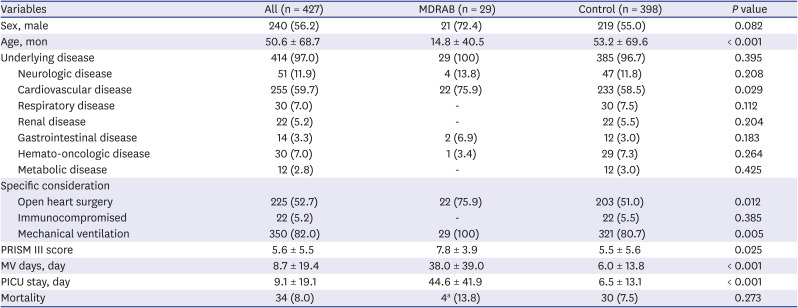
Clinical characteristics of patients
Table 3
Clinical characteristics and outcomes of the patients in the MDRAB group and comparisons with the control group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download