INTRODUCTION
MATERIALS AND METHODS
Animals and diet
Preparation of nuclear fractions
Western blotting
Quantitative polymerase chain reaction (qPCR)
Enzyme-linked immunosorbent assay (ELISA)
Statistical analysis
RESULTS
Changes in spleen weights in HFCD-fed mice
 | Fig. 1Effects of PEITC and DIM supplementation on spleen weight and images of representative spleen tissues from mice fed a high fat/cholesterol diet. (A) Summary of spleen weight in each group. (B) Representative macroscopic images of dissected spleens from each group. There was no significant difference in spleen weights. Data presented as means ± SD (n = 10); different letters indicate significant differences (P < 0.05), as determined by applying Duncan's multiple range test. CON: AIN-93G diet, HFCD: 60% calories from fat+1% cholesterol, HFCD+P30: HFCD+30 mg/kg/day of PEITC, HFCD+P75: HFCD+75 mg/kg/day of PEITC, HFCD+D1.5: HFCD+1.5 mg/kg/day of DIM, HFCD+D7.5: HFCD+7.5 mg/kg/day of DIM.PEITC, phenethyl isothiocyanate; HFCD, high fat/cholesterol diet; DIM, 3,3′-diindolylmethane.
|
Suppression of IL-6 secretion in serum by PEITC and DIM supplementation in HFCD-fed mice
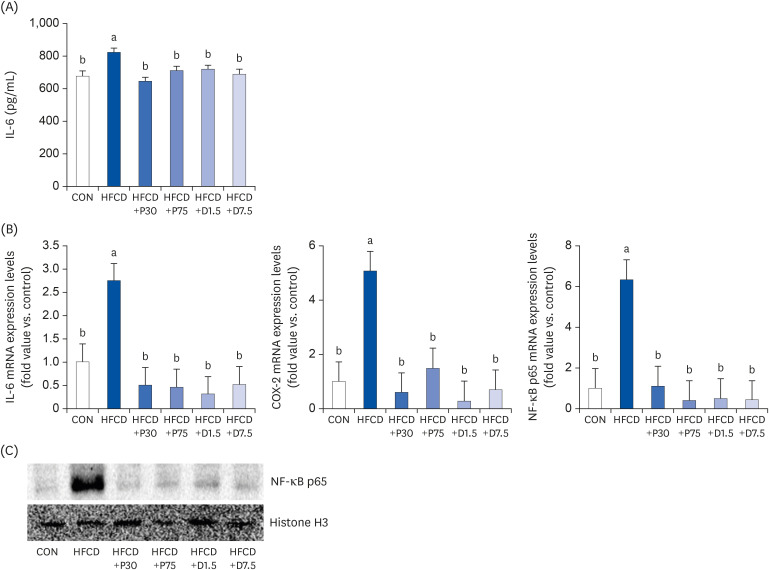 | Fig. 2PEITC and DIM supplements suppress IL-6 secretion, IL-6 mRNA, COX-2 mRNA, and NF-κB p65 mRNA expressions and NF-κB p65 gene expression in serum and spleen of high fat/cholesterol diet-fed mice. (A) Pro-inflammatory IL-6 secretion was determined by enzyme-linked immunosorbent assay. Total RNA was subjected to qPCR analysis, as described in the materials and methods section. (B) Results of qPCR analysis of IL-6, COX-2, and NF-κB p65 gene expression in high fat/cholesterol diet-fed mice. The transcript mRNA levels of IL-6, COX-2, and NF-κB p65 were normalized to that of β-actin. (C) Lysates were prepared and underwent western blotting with NF-κB p65 antibody, as described in the materials and methods section. Increased NF-κB p65 expression was confirmed via western blot analysis. Values were analyzed using the 2-∆∆CT method. Significance was determined by comparison with β-actin normalized 2-∆∆CT values. Data presented as means ± SD (n = 3), and different letters indicate significant differences (P < 0.05, a > b), as determined by applying Duncan's multiple range test. CON: AIN-93G diet, HFCD: 60% calories from fat + 1% cholesterol, HFCD+P30: HFCD+30 mg/kg/day of PEITC, HFCD+P75: HFCD+75 mg/kg/day of PEITC, HFCD+D1.5: HFCD+1.5 mg/kg/day of DIM, HFCD+D7.5: HFCD+7.5 mg/kg/day of DIM.IL-6, interleukin 6; COX-2, cyclooxygenase 2; PEITC, phenethyl isothiocyanate; DIM, 3,3′-diindolylmethane; HFCD, high fat/cholesterol diet; NF-κB, nuclear factor kappa B; qPCR, quantitative polymerase chain reaction.
|
Inhibition of inflammatory protein expressions in spleen by PEITC and DIM supplementation in HFCD-fed mice
Inhibition of TLR2/4 and MyD88 mRNA expression levels in spleen by PEITC and DIM supplementation in HFCD-fed mice
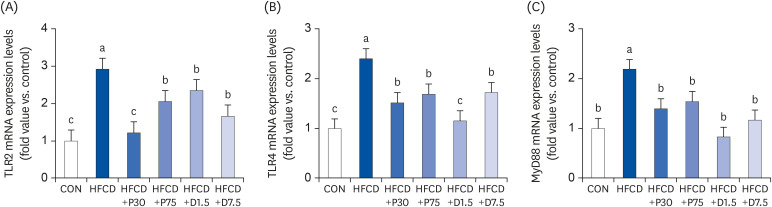 | Fig. 3PEITC and DIM suppress (A) TLR2, (B) TLR4, and (C) MyD88 mRNA expressions in the spleen of high fat/cholesterol diet-fed mice. Results of qPCR analysis of TLR2, TLR4, and MyD88 gene expression in high fat/cholesterol diet-fed mice. Total RNA was subjected to qPCR analysis, as described in the materials and methods section. The transcript mRNA levels of TLR2, TLR4, and MyD88 were normalized to that of β-actin. Values were analyzed using the 2-∆∆CT method. Significance was determined by comparison with β-actin normalized 2-∆∆CT values. Data presented as means ± SD (n = 3), and different letters indicate significant differences (P < 0.05, a > b > c), as determined by applying Duncan's multiple range test. CON: AIN-93G diet, HFCD: 60% calories from fat+1% cholesterol, HFCD+P30: HFCD+30 mg/kg/day of PEITC, HFCD+P75: HFCD+75 mg/kg/day of PEITC, HFCD+D1.5: HFCD+1.5 mg/kg/day of DIM, HFCD+D7.5: HFCD+7.5 mg/kg/day of DIM.TLR, Toll-like receptor; HFCD, high fat/cholesterol diet; MyD88, myeloid differentiation primary response 88; DIM, 3,3′-diindolylmethane; PEITC, phenethyl isothiocyanate; qPCR, quantitative polymerase chain reaction.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download