1. Braverman PK. Premenstrual syndrome and premenstrual dysphoric disorder. J Pediatr Adolesc Gynecol. 2007; 20:3–12. PMID:
17289510.

2. Hong JP, Park S, Wang HR, Chang SM, Sohn JH, Jeon HJ, Lee HW, Cho SJ, Kim BS, Bae JN, Cho MJ. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012; 47:1937–1945. PMID:
22538387.

3. Deuster PA, Adera T, South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999; 8:122–128. PMID:
10101982.

4. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016; 94:236–240. PMID:
27479626.
5. Pearlstein TB. Hormones and depression: what are the facts about premenstrual syndrome, menopause, and hormone replacement therapy? Am J Obstet Gynecol. 1995; 173:646–653. PMID:
7645647.

6. Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, Rubinow DR. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017; 174:980–989. PMID:
28427285.

7. Yen JY, Wang PW, Su CH, Liu TL, Long CY, Ko CH. Estrogen levels, emotion regulation, and emotional symptoms of women with premenstrual dysphoric disorder: the moderating effect of estrogen receptor 1α polymorphism. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 82:216–223. PMID:
29146473.

8. Osório FL, de Paula Cassis JM, Machado de Sousa JP, Poli-Neto O, Martín-Santos R. Sex hormones and processing of facial expressions of emotion: a systematic literature review. Front Psychol. 2018; 9:529. PMID:
29695991.

9. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000; 80:1523–1631. PMID:
11015620.
10. Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci. 2013; 6:168–175. PMID:
24347930.

11. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015; 15:217–230. PMID:
25743222.

12. Lennartsson AK, Jonsdottir IH. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology. 2011; 36:1530–1539. PMID:
21621331.

13. Pałubska S, Adamiak-Godlewska A, Winkler I, Romanek-Piva K, Rechberger T, Gogacz M. Hyperprolactinaemia - a problem in patients from the reproductive period to the menopause. Przegl Menopauz. 2017; 16:1–7.

14. Seidlova-Wuttke D, Wuttke W. The premenstrual syndrome, premenstrual mastodynia, fibrocystic mastopathy and infertility have often common roots: effects of extracts of chasteberry (
Vitex agnus castus) as a solution. Clin Phytoscience. 2017; 3:6.

15. Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and autoimmunity. Front Immunol. 2018; 9:73. PMID:
29483903.

16. Wang X, Ma L, Zhang EJ, Zou JL, Guo H, Peng SW, Wu JH. Water extract of Fructus Hordei Germinatus shows antihyperprolactinemia activity via dopamine D2 receptor. Evid Based Complement Alternat Med. 2014; 2014:579054. PMID:
25254056.
17. Li MX, Liu H, Li Y, Wang F, Zhang PR, Zang P. Anti-hyperprolactinemic effect of formula malt decoction, a Chinese herbal cocktail. Trop J Pharm Res. 2015; 14:263–269.

18. Kim AR, Kim HS, Kim DK, Lee JH, Yoo YH, Kim JY, Park SK, Nam ST, Kim HW, Park YH, Lee D, Lee MB, Kim YM, Choi WS. The extract of
Chrysanthemum zawadskii var.
latilobum ameliorates collagen-induced arthritis in mice. Evid Based Complement Alternat Med. 2016; 2016:3915013. PMID:
27840652.
19. Kim KY, Oh TW, Yang HJ, Kim YW, Ma JY, Park KI. Ethanol extract of
Chrysanthemum zawadskii Herbich induces autophagy and apoptosis in mouse colon cancer cells through the regulation of reactive oxygen species. BMC Complement Altern Med. 2019; 19:274. PMID:
31638961.

20. Song HS, Kwon JE, Baek HJ, Kim CW, Jeon H, Ra JS, Lee HK, Kang SC. Sorghum fermented by
Aspergillus oryzae NK enhances inhibition of vascular inflammation in TNF-α-stimulated human aortic smooth muscle cells. Int J Vitam Nutr Res. 2018; 88:309–318. PMID:
31237194.
21. Skwarło-Sońta K. Prolactin as an immunoregulatory hormone in mammals and birds. Immunol Lett. 1992; 33:105–121. PMID:
1446915.

22. Pereira Suarez AL, López-Rincón G, Martínez Neri PA, Estrada-Chávez C. Prolactin in inflammatory response. Adv Exp Med Biol. 2015; 846:243–264. PMID:
25472542.
23. McCallum RW, Sowers JR, Hershman JM, Sturdevant RA. Metoclopramide stimulates prolactin secretion in man. J Clin Endocrinol Metab. 1976; 42:1148–1152. PMID:
777023.

24. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001; 22:724–763. PMID:
11739329.

25. Klein-Laansma CT, Jong M, von Hagens C, Jansen JP, van Wietmarschen H, Jong MC. Semi-individualized homeopathy add-on versus usual care only for premenstrual disorders: a randomized, controlled feasibility study. J Altern Complement Med. 2018; 24:684–693. PMID:
29565636.

26. Verkaik S, Kamperman AM, van Westrhenen R, Schulte PF. The treatment of premenstrual syndrome with preparations of
Vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017; 217:150–166. PMID:
28237870.
27. Zamani M, Neghab N, Torabian S. Therapeutic effect of
Vitex agnus castus in patients with premenstrual syndrome. Acta Med Iran. 2012; 50:101–106. PMID:
22359078.
28. Silva LG, Ferguson BS, Faciola AP. Rapid communication: prolactin and hydrocortisone impact TNFα-mediated mitogen-activated protein kinase signaling and inflammation of bovine mammary epithelial (MAC-T) cells. J Anim Sci. 2017; 95:5524–5531. PMID:
29293766.
29. Cerqueira RO, Frey BN, Leclerc E, Brietzke E.
Vitex agnus castus for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Women Ment Health. 2017; 20:713–719.
30. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007; 15:252–259. PMID:
18236016.

31. Moreno-Carranza B, Bravo-Manríquez M, Baez A, Ledesma-Colunga MG, Ruiz-Herrera X, Reyes-Ortega P, de Los Ríos EA, Macotela Y, Martínez de la Escalera G, Clapp C. Prolactin regulates liver growth during postnatal development in mice. Am J Physiol Regul Integr Comp Physiol. 2018; 314:R902–8. PMID:
29466685.

32. Rondeau G, Merouani A, Phan V, Deal C, Robitaille P. Serum prolactin levels in a uremic child: effects of bilateral nephrectomy and kidney transplantation. NDT Plus. 2011; 4:303–306. PMID:
25984175.

33. Gunin AG, Emelianov V, Tolmachev AS, Tolmacheva A. Effect of prolactin and dopaminergic drugs on uterine response to chronic estrogen exposure. J Endocrinol. 2002; 172:61–69. PMID:
11786374.

34. Holm P, Shalmi M, Korsgaard N, Guldhammer B, Skouby SO, Stender S. A partial estrogen receptor agonist with strong antiatherogenic properties without noticeable effect on reproductive tissue in cholesterol-fed female and male rabbits. Arterioscler Thromb Vasc Biol. 1997; 17:2264–2272. PMID:
9351399.

35. Orkhon B, Kobayashi K, Javzan B, Sasaki K. Astragalus root induces ovarian β‑oxidation and suppresses estrogen‑dependent uterine proliferation. Mol Med Rep. 2018; 18:5198–5206. PMID:
30272268.

36. Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007; 282:11613–11617. PMID:
17329241.

37. Fischer DP, Griffiths AL, Lui S, Sabar UJ, Farrar D, O'Donovan PJ, Woodward DF, Marshall KM. Distribution and function of prostaglandin E
2 receptors in mouse uterus: translational value for human reproduction. J Pharmacol Exp Ther. 2020; 373:381–390. PMID:
32205366.
38. Koshikawa N, Tatsunuma T, Furuya K, Seki K. Prostaglandins and premenstrual syndrome. Prostaglandins Leukot Essent Fatty Acids. 1992; 45:33–36. PMID:
1546064.



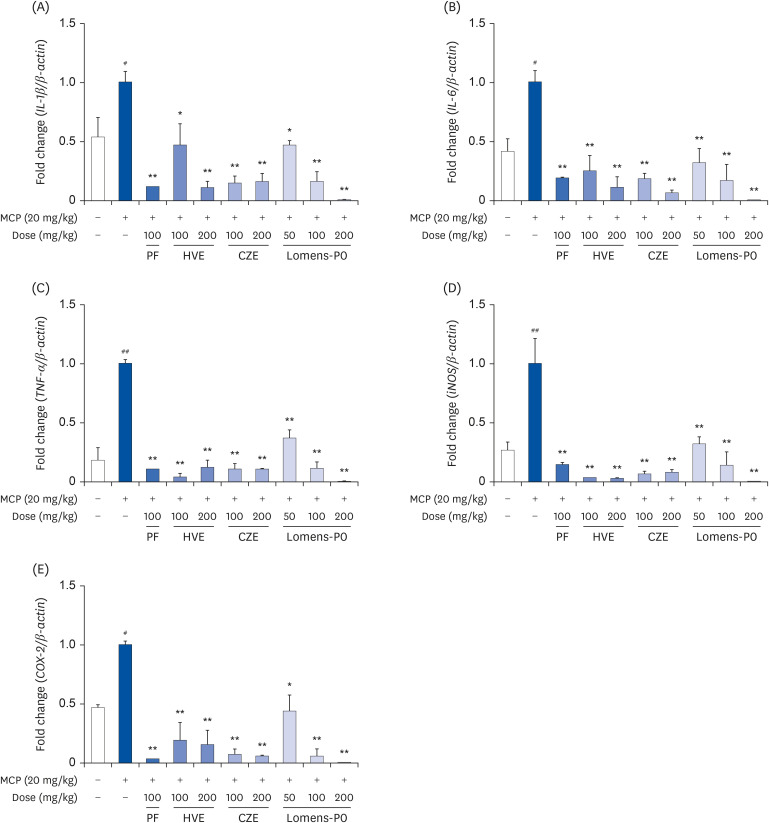
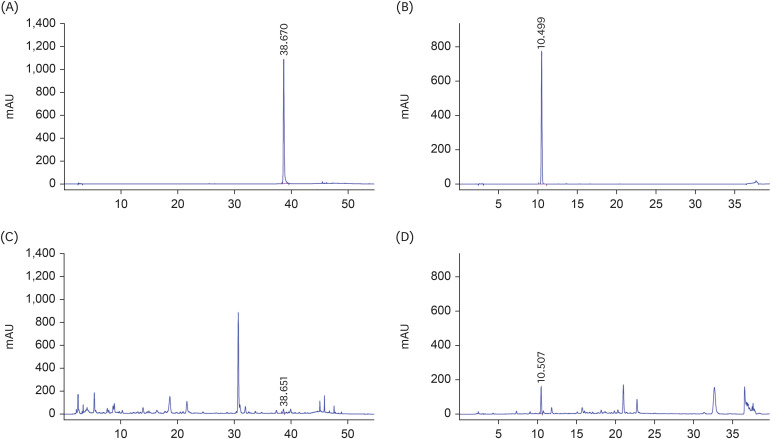
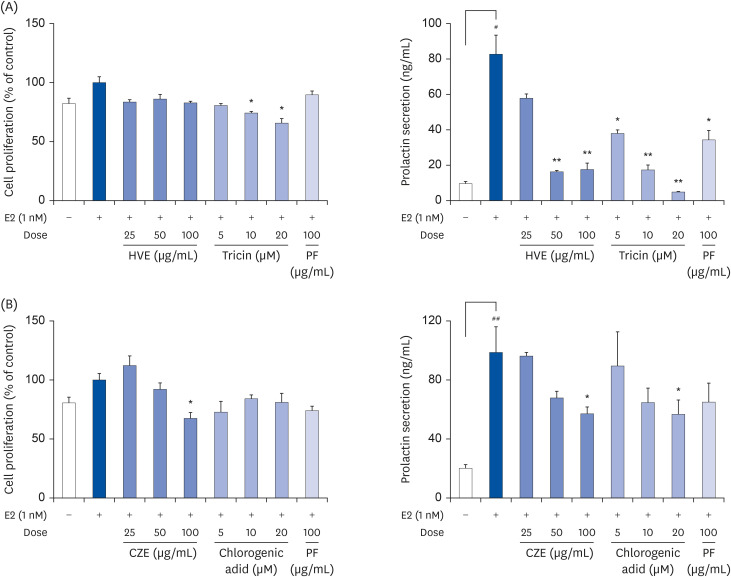
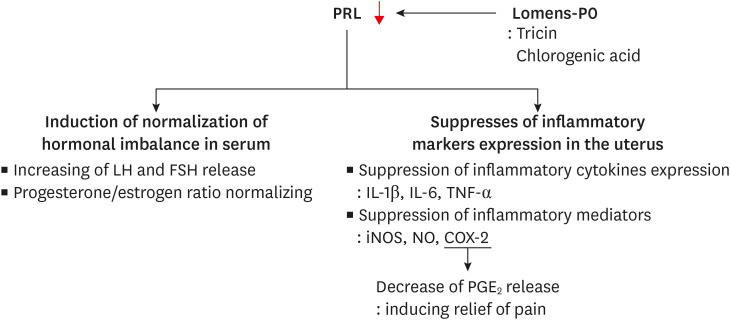




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download