Abstract
Objectives
This study investigated the indirect effect of calcium-enriched mixture (CEM) cement and mineral trioxide aggregate (MTA), as 2 calcium silicate-based hydraulic cements, on human dental pulp stem cells (hDPSCs) through different dentin thicknesses.
Materials and Methods
Two-chamber setups were designed to simulate indirect pulp capping (IPC). Human molars were sectioned to obtain 0.1-, 0.3-, and 0.5-mm-thick dentin discs, which were placed between the 2 chambers to simulate an IPC procedure. Then, MTA and CEM were applied on one side of the discs, while hDPSCs were cultured on the other side. After 2 weeks of incubation, the cells were removed, and cell proliferation, morphology, and attachment to the discs were evaluated under scanning electron microscopy (SEM). Energy-dispersive X-ray (EDXA) spectroscopy was performed for elemental analysis. Alkaline phosphatase (ALP) activity was assessed quantitatively. The data were analyzed using the Kruskal-Wallis and Mann-Whitney tests.
Results
SEM micrographs revealed elongated cells, collagen fibers, and calcified nucleations in all samples. EDXA verified that the calcified nucleations consisted of calcium phosphate. The largest calcifications were seen in the 0.1-mm-thick dentin subgroups. There was no significant difference in ALP activity across the CEM subgroups; however, ALP activity was significantly lower in the 0.1-mm-thick dentin subgroup than in the other MTA subgroups (p < 0.05).
Conclusions
The employed capping biomaterials exerted biological activity on hDPSCs, as shown by cell proliferation, morphology, and attachment and calcific precipitations, through 0.1- to 0.5-mm-thick layers of dentin. In IPC, the bioactivity of these endodontic biomaterials is probably beneficial.
References
1. Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008; 139:705–712.
2. Schwendicke F, Frencken JE, Bjørndal L, Maltz M, Manton DJ, Ricketts D, Van Landuyt K, Banerjee A, Campus G, Doméjean S, Fontana M, Leal S, Lo E, Machiulskiene V, Schulte A, Splieth C, Zandona AF, Innes NP. Managing carious lesions: consensus recommendations on carious tissue removal. Adv Dent Res. 2016; 28:58–67.
3. Ricketts D. Management of the deep carious lesion and the vital pulp dentine complex. Br Dent J. 2001; 191:606–610.

4. Chogle SM, Goodis HE, Kinaia BM. Pulpal and periradicular response to caries: current management and regenerative options. Dent Clin North Am. 2012; 56:521–536.
5. Gruythuysen RJ, van Strijp AJ, Wu MK. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J Endod. 2010; 36:1490–1493.

6. Asgary S, Hassanizadeh R, Torabzadeh H, Eghbal MJ. Treatment outcomes of 4 vital pulp therapies in mature molars. J Endod. 2018; 44:529–535.

8. Asgary S, Eghbal MJ, Bagheban AA. Long-term outcomes of pulpotomy in permanent teeth with irreversible pulpitis: a multi-center randomized controlled trial. Am J Dent. 2017; 30:151–155.
9. Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S. Treatment outcomes of primary molars direct pulp capping after 20 months: a randomized controlled trial. Iran Endod J. 2013; 8:149–152.
10. Araújo LB, Cosme-Silva L, Fernandes AP, Oliveira TM, Cavalcanti BD, Gomes Filho JE, Sakai VT. Effects of mineral trioxide aggregate, Biodentine™and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J Appl Oral Sci. 2018; 26:e20160629.

11. Agrafioti A, Taraslia V, Chrepa V, Lymperi S, Panopoulos P, Anastasiadou E, Kontakiotis EG. Interaction of dental pulp stem cells with Biodentine and MTA after exposure to different environments. J Appl Oral Sci. 2016; 24:481–486.

12. Kamali A, Javadpour S, Javid B, Kianvash Rad N, Naddaf Dezfuli S. Effects of chitosan and zirconia on setting time, mechanical strength, and bioactivity of calcium silicate-based cement. Int J Appl Ceram Technol. 2017; 14:135–144.

13. Kim M, Kim S, Ko H, Song M. Effect of ProRoot MTA® and Biodentine® on osteoclastic differentiation and activity of mouse bone marrow macrophages. J Appl Oral Sci. 2019; 27:e20180150.

14. Moazzami F, Ghahramani Y, Tamaddon AM, Dehghani Nazhavani A, Adl A. A histological comparison of a new pulp capping material and mineral trioxide aggregate in rat molars. Iran Endod J. 2014; 9:50–55.
15. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.

16. Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010; 43:565–571.

17. Tak O, Usumez A. Diffusion of HEMA from resin cements through different dentin thicknesses in vitro. Am J Dent. 2015; 28:285–291.
18. Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. 2002; 15:41–46.
19. Asgary S, Nazarian H, Khojasteh A, Shokouhinejad N. Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J Endod. 2014; 40:387–392.

20. Rahimi S, Mokhtari H, Shahi S, Kazemi A, Asgary S, Eghbal MJ, Mesgariabbasi M, Mohajeri D. Osseous reaction to implantation of two endodontic cements: mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). Med Oral Patol Oral Cir Bucal. 2012; 17:e907–e911.

21. Asgary S, Eghbal MJ, Ehsani S. Periradicular regeneration after endodontic surgery with calcium-enriched mixture cement in dogs. J Endod. 2010; 36:837–841.

22. Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010; 36:1778–1781.

23. Asgary S, Parirokh M, Eghbal MJ, Ghoddusi J. SEM evaluation of pulp reaction to different pulp capping materials in dog's teeth. Iran Endod J. 2006; 1:117–123.
24. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005; 31:97–100.

25. Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009; 35:147–152.

26. Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent. 2007; 35:636–642.

27. Aubin JE, Triffitt JT. Chapter 4 – mesenchymal stem cells and osteoblast differentiation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. 2nd ed.San Diego, CA: Academic Press;2002. p59-81.
28. Kim MJ, Kim KN, Lee YK, Kim KM. Cytotoxicity test of dentin bonding agents using millipore filters as dentin substitutes in a dentin barrier test. Clin Oral Investig. 2013; 17:1489–1496.

29. Sengün A, Yalçın M, Ülker HE, Öztürk B, Hakkı SS. Cytotoxicity evaluation of dentin bonding agents by dentin barrier test on 3-dimensional pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e83–e88.
30. Dunne SM. The limitation of visual perception in restorative dentistry. Dent Update. 1993; 20:198–201.
Figure 1.
The setup for the experiment. (A) Separated view; (B) Assembled view. The setup had 1) 2 plexiglass rectangular chambers for the placement of materials/cells and 2) a hole (red arrowhead) for placement of the dentin discs.
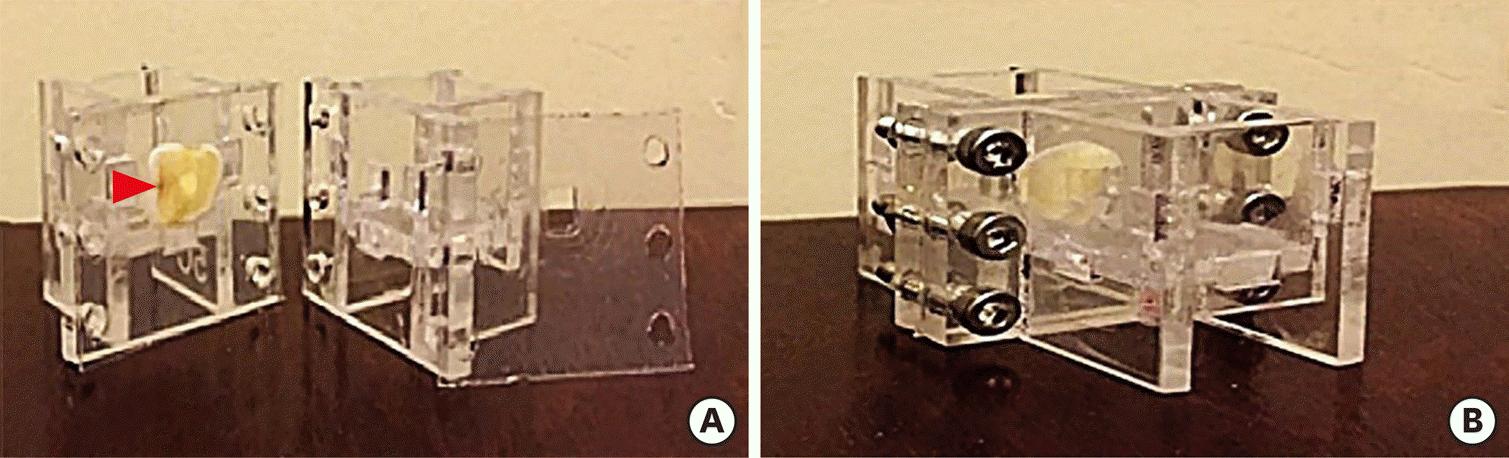
Figure 2.
Scanning electron micrographs of calcium-enriched mixture cement (left column) with (A) 0.1 mm, (C) 0.3 mm, and (E) 0.5 mm dentin thicknesses and mineral trioxide aggregate cement (right column) with (B) 0.1 mm, (D) 0.3 mm, and (F) 0.5 mm dentin thicknesses. White arrowheads indicate elongated cells and blue arrow heads show calcified nodules.
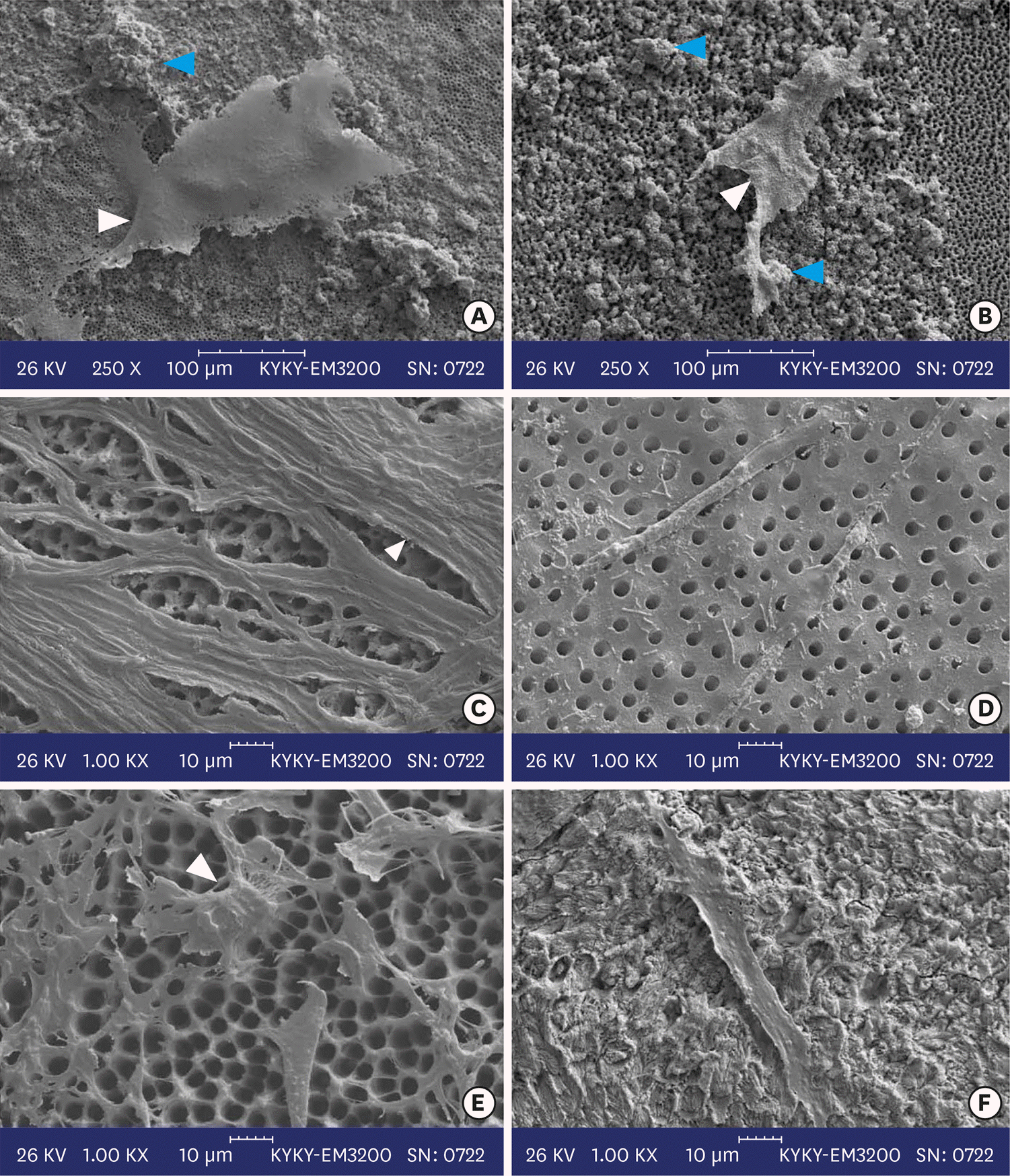
Figure 3.
Energy-dispersive X-ray spectroscopy of the (A) Calcium-enriched mixture and (B) Mineral trioxide aggregate groups with 0.1-mm-thick dentin. Calcium and phosphorus ions were the predominant ions in the experimental groups.
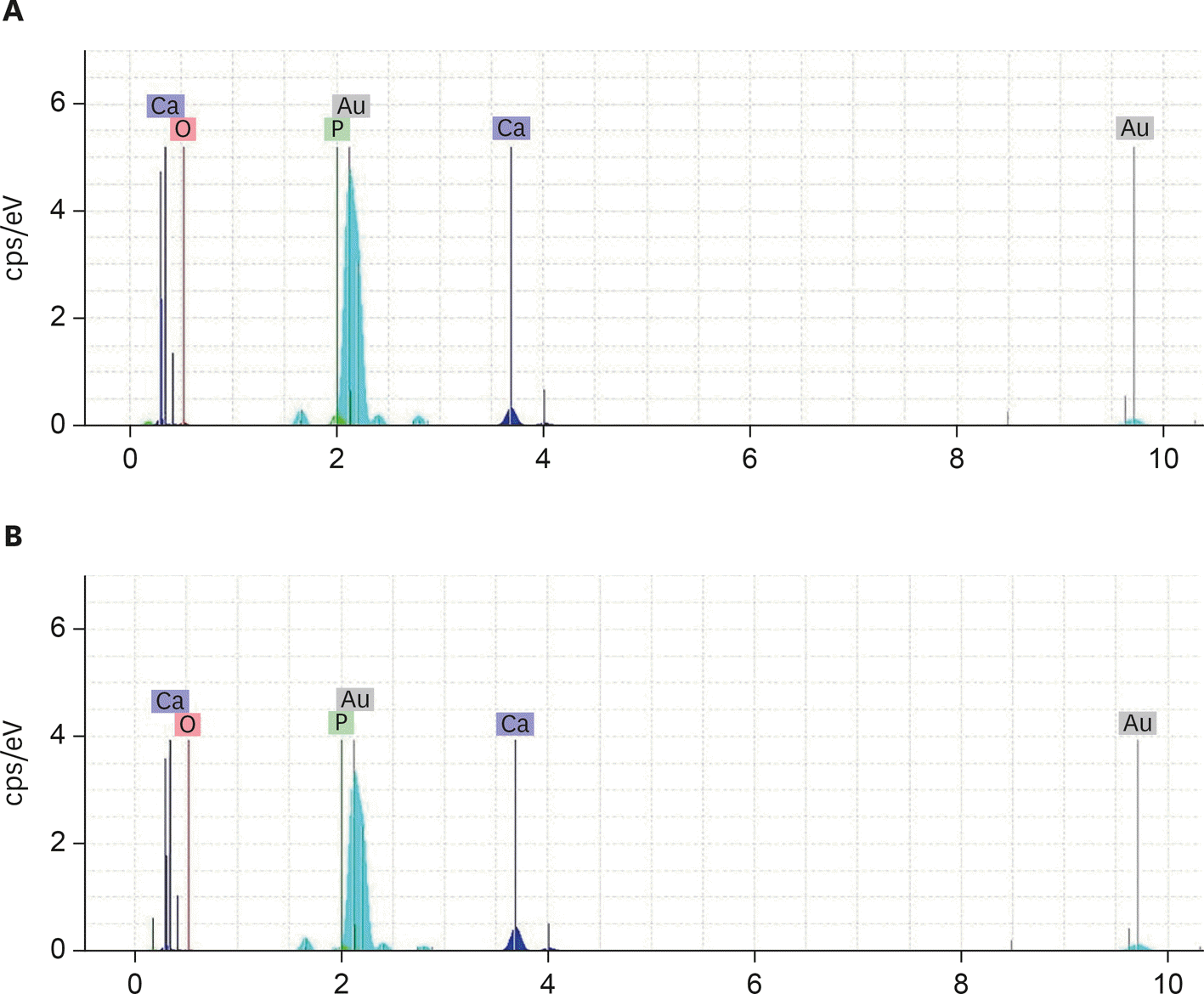
Table 1.
Alkaline phosphatase (ALP) activity at various concentrations
The values are presented as mean ± standard deviation, and the numbers in parentheses are the mean ranks in the group. Activity 30, activity 40, activity 50, activity 60, and activity 80 refer to 30%, 40%, 50%, 60%, and 80% levels of ALP activity, respectively.
Different lowercase letters in the same column indicate the presence of statistically significant differences.
CEM, calcium-enriched mixture; MTA, mineral trioxide aggregate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download