Abstract
Fast and accurate detection of viral RNA pathogens is important in apiculture. A polymerase chain reaction (PCR)-based detection method has been developed, which is simple, specific, and sensitive. In this study, we rapidly (in 1 min) synthesized cDNA from the RNA of deformed wing virus (DWV)-infected bees (Apis mellifera), and then, within 10 min, amplified the target cDNA by ultra-rapid qPCR. The PCR products were hybridized to a DNA-chip for confirmation of target gene specificity. The results of this study suggest that our method might be a useful tool for detecting DWV, as well as for the diagnosis of RNA virus-mediated diseases on-site.
References
1. McMenamin AJ, Genersch E. Honey bee colony losses and associated viruses. Curr Opin Insect Sci. 2015; 8:121–129.

2. Genersch E, Yue C, Fries I, de Miranda JR. Detection of Deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. J Invertebr Pathol. 2006; 91:61–63.
3. Tehel A, Brown MJ, Paxton RJ. Impact of managed honey bee viruses on wild bees. Curr Opin Virol. 2016; 19:16–22.

4. Reddy KE, Noh JH, Yoo MS, Kim YH, Kim NH, Doan HT, Ramya M, Jung SC, Van Quyen D, Kang SW. Molecular characterization and phylogenetic analysis of deformed wing viruses isolated from South Korea. Vet Microbiol. 2013; 167:272–279.

5. Tentcheva D, Gauthier L, Jouve S, Canabady-Rochelle L, Dainat B, Cousserans F, Colina ME, Ballb BV, Bergoin M. Polymerase chain reaction detection of deformed wing virus (DWV) in Apis mellifera and Varroa destructor. Apidologie (Celle). 2004; 35:431–439.
6. Chen YP, Higgins JA, Feldlaufer MF. Quantitative real-time reverse transcription-PCR analysis of deformed wing virus infection in the honeybee (Apis mellifera L.). Appl Environ Microbiol. 2005; 71:436–441.
7. Yoo MS, Thi KC, Van Nguyen P, Han SH, Kwon SH, Yoon BS. Rapid detection of sacbrood virus in honeybee using ultra-rapid real-time polymerase chain reaction. J Virol Methods. 2012; 179:195–200.

8. Jamnikar Cigleneč ki U, Toplak I. Development of a real-time RT-PCR assay with TaqMan probe for specific detection of acute bee paralysis virus. J Virol Methods. 2012; 184:63–68.

9. Haddad NJ, Noureddine A, Al-Shagour B, Loucif-Ayad W, El-Niweiri MA, Anaswah E, Hammour WA, El-Obeid D, Imad A, Shebl MA, Almaleky AS, Nasher A, Walid N, Bergigui MF, Yañez O, de Miranda JR. Distribution and variability of deformed wing virus of honeybees (Apis mellifera) in the Middle East and North Africa. Insect Sci. 2017; 24:103–113.
10. Kukielka D, Esperón F, Higes M, Sánchez-Vizcaíno JM. A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J Virol Methods. 2008; 147:275–281.
11. Lim HY. Development of novel rapid detection method for deformed wing virus (DWV) using ultra-fast high-performance PCR (UF-HP PCR). J Apic. 2013; 28:237–244.
12. Ma M, Ma C, Li M, Wang S, Yang S, Wang S. Loop-mediated isothermal amplification for rapid detection of Chinese sacbrood virus. J Virol Methods. 2011; 176:115–119.

13. Yoo MS, Noh JH, Yoon BS, Reddy KE, Kweon CH, Jung SC, Kang SW. Reverse transcription loop-mediated isothermal amplification for sensitive and rapid detection of Korean sacbrood virus. J Virol Methods. 2012; 186:147–151.

14. Han SH, Lee DB, Lee DW, Kim EH, Yoon BS. Ultra-rapid real-time PCR for the detection of Paenibacillus larvae, the causative agent of American Foulbrood (AFB). J Invertebr Pathol. 2008; 99:8–13.
15. Lee DW, Kim EH, Yoo MS, Han SH. Ultra-rapid real-time PCR for the detection of human immunodeficiency virus (HIV). Korean J Microbiol. 2007; 43:91–99.
16. Lim SJ, Kim JM, Lee CW, Yoon BS. Development of ultra-rapid multiplex PCR detection against 6 major pathogens in honeybee. J Apic. 2017; 32:27–39.

17. Lung O, Fisher M, Beeston A, Hughes KB, Clavijo A, Goolia M, Pasick J, Mauro W, Deregt D. Multiplex RT-PCR detection and microarray typing of vesicular disease viruses. J Virol Methods. 2011; 175:236–245.

18. Choe SE, Nguyen LT, Noh JH, Koh HB, Jean YH, Kweon CH, Kang SW. Prevalence and distribution of six bee viruses in Korean Apis cerana populations. J Invertebr Pathol. 2012; 109:330–333.
19. Tantillo G, Bottaro M, Di Pinto A, Martella V, Di Pinto P, Terio V. Virus infections of honeybees Apis Mellifera. Ital J Food Saf. 2015; 4:5364.
20. Cai HY, Caswell JL, Prescott JF. Nonculture molecular techniques for diagnosis of bacterial disease in animals: a diagnostic laboratory perspective. Vet Pathol. 2014; 51:341–350.
Fig. 1.
Location of primers for URRT-qPCR and probes for DNA-chip. The amplified target region is located at 9060–9282 bp on DWV RNA-dependent RNA polymerase. The size of the amplified target was 223 bp. The four DWV-specific probes represent the PCR product applied to the DNA-chip. DWV, deformed wing virus; PCR, polymerase chain reaction; URRT-qPCR, ultra-rapid reverse transcription quantitative polymerase chain reaction.
Fig. 2.
Comparison of the effectiveness of different reverse transcription reaction times. (A) Cts were compared to determine the efficiencies of cDNA synthesis with different RT reaction times in one-step or two-step RT-qPCR. (B) In one-step or two-step RT-qPCR, the Ct time was analyzed depending on the cDNA synthesis reaction time. Data are presented as mean ± strandard deviation values of three independent experiments. RT-PCR, reverse transcription-polymerase chain reaction; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
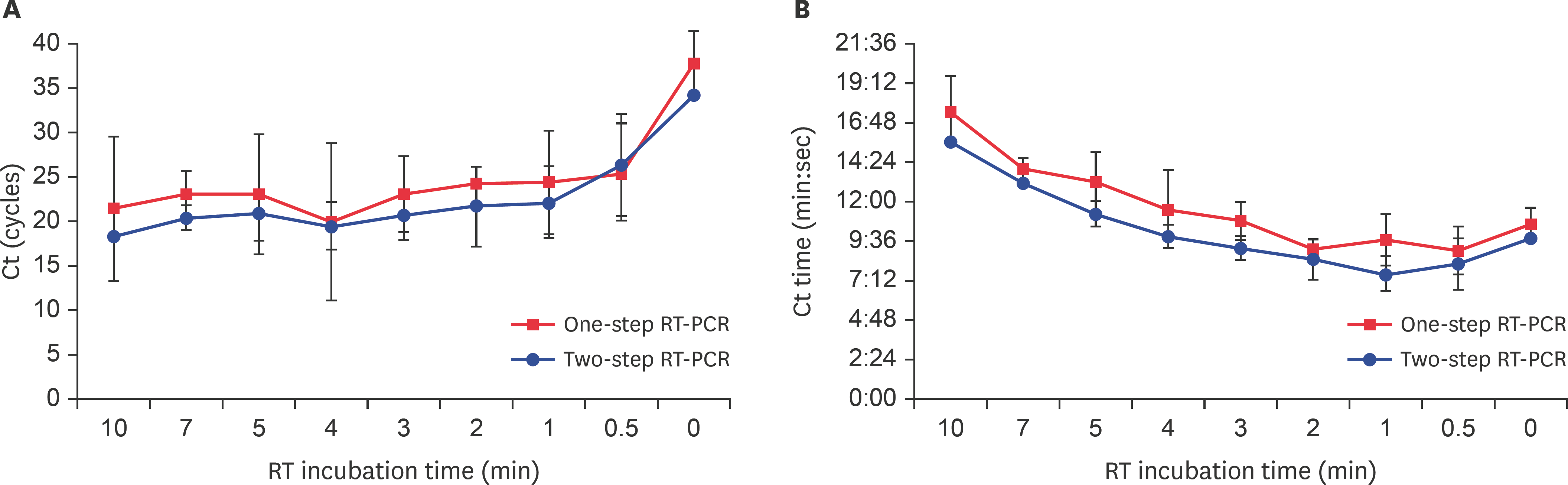
Fig. 3.
Optimal conditions for each step of DWV-specific URRT-qPCR. URRT-qPCR conditions were evaluated by examining Ct time values versus (A) annealing temperature, (B) annealing time, and (C) polymerization time. The annealing time was determined based on the stability of the detection system. Data are presented as mean ± strandard deviation values of three independent experiments. DWV, deformed wing virus; URRT-qPCR, ultra-rapid reverse transcription quantitative polymerase chain reaction.
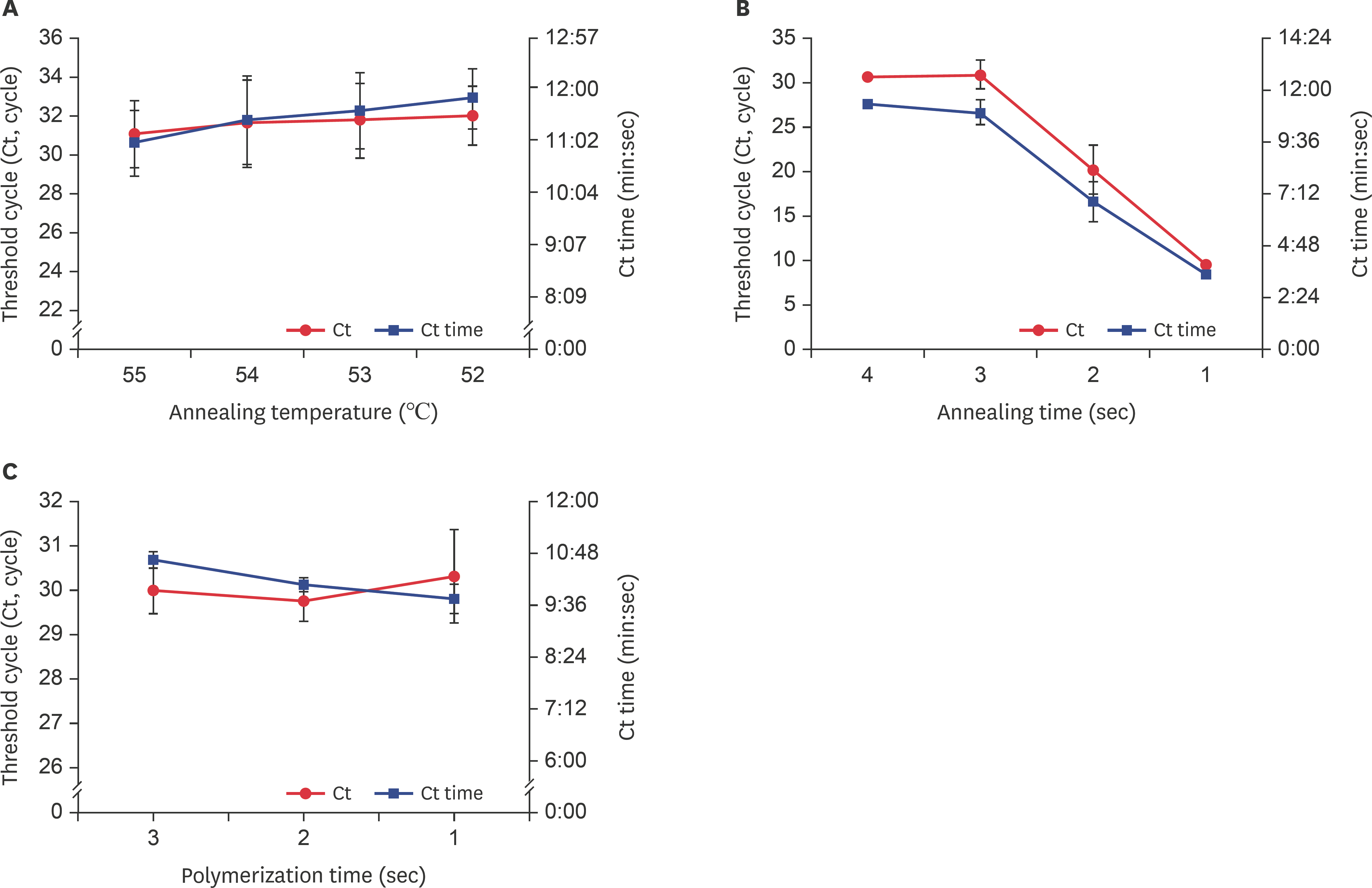
Fig. 4.
Detection of DWV-specific target gene using URRT-qPCR. (A) DWV-specific URRT-qPCR was performed using recombinant DNA. The fluorescence signals of the amplified target gene were calculated to determine detection limits. Construction of a standard curve and (B) melting point analysis show that it differed the Tm valuese between specific and nonspecific amplicon. (C) The DWV-specific URRT-qPCR was performed with local samples and recombinant DNA, and it shows that Yeosu and Suwon sample have different values of Tm by melting point analysis. (D) For DWV-infected honeybee samples, we calculated the number of DWV molecules via regression analysis of the amplified target gene product. DWV, deformed wing virus; URRT-qPCR, ultra-rapid reverse transcription quantitative polymerase chain reaction.
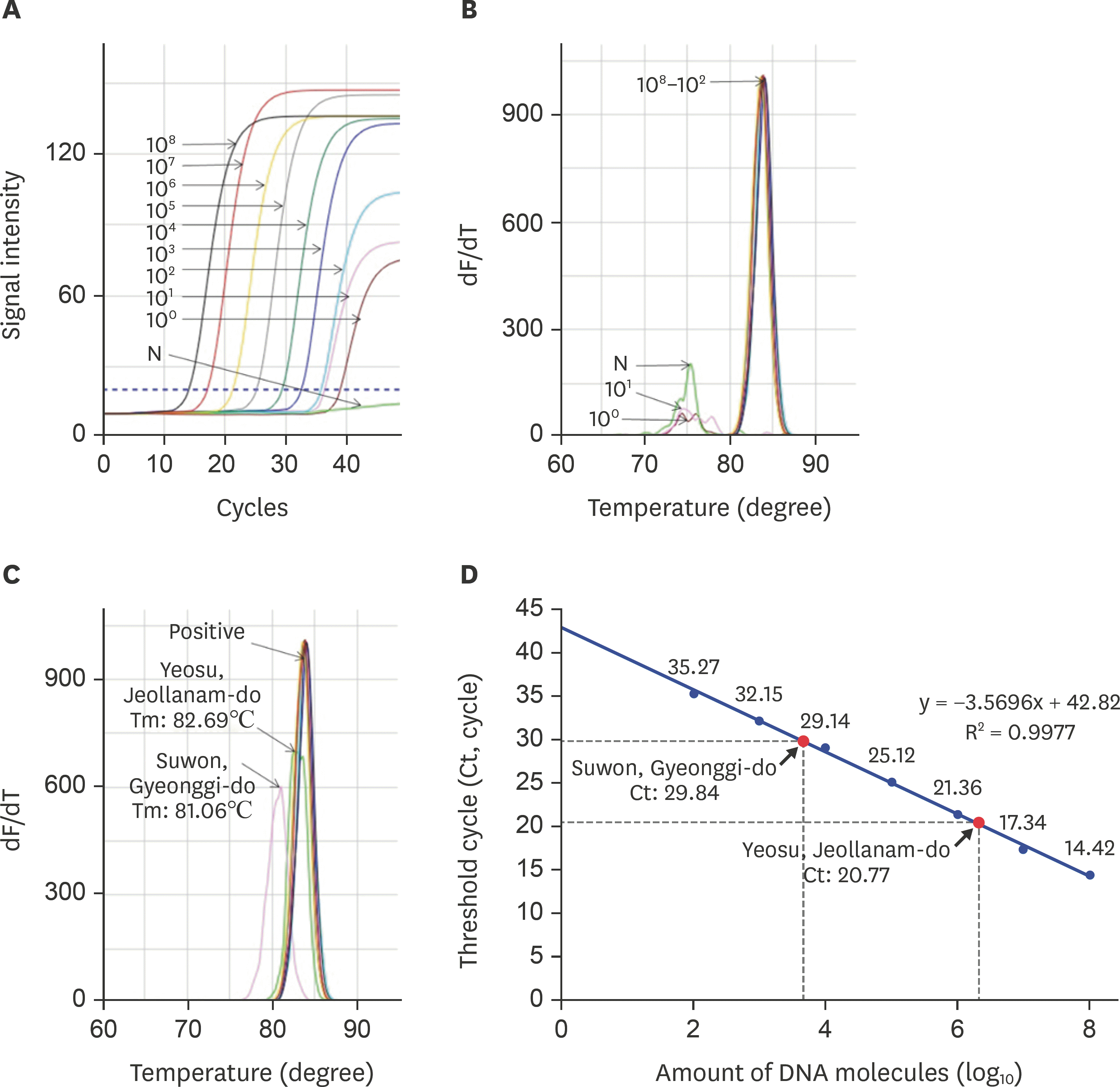
Fig. 5.
Confirmation of amplified target gene presence on a DNA-chip. (A) Amplification of the target gene was confirmed on a DNA-chip by using four deformed wing virus-specific probes. The bar graph shows the SBR values, according to the number of recombinant DNA molecules. The SBR in each probe hybridized with deformed wing virus specific gene are shown. (B) Each sample was hybridized to each of the specific probes. The SBR values are shown by the graph. SBR, spot/ background ratio.
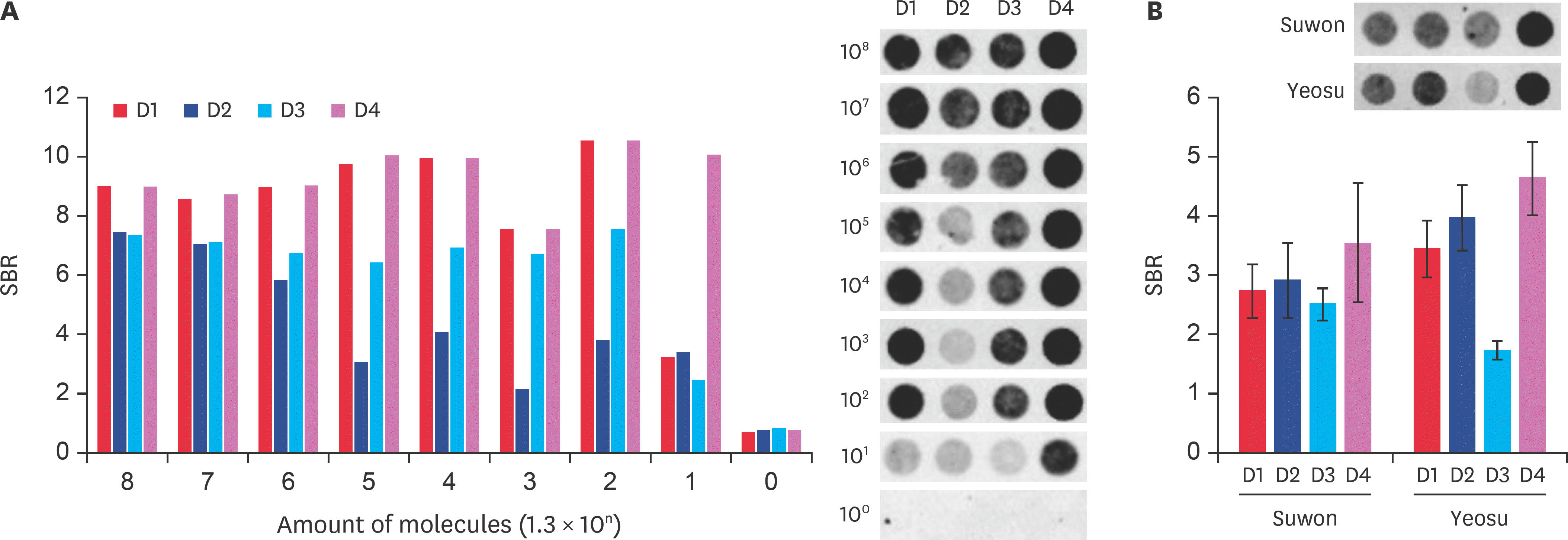
Table 1.
List of nucleotide sequences of the primers and probes used in this study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download