Abstract
Bovine ephemeral fever virus (BEFV) causes bovine ephemeral fever, which can produce considerable economic damage to the cattle industry. However, there is limited experimental evidence regarding the underlying mechanisms of BEFV. Annexin A2 (AnxA2) is a calcium and lipid-conjugated protein that binds phospholipids and the cytoskeleton in a Ca2+-dependent manner, and it participates in various cellular functions, including vesicular trafficking, organization of membrane domains, and virus proliferation. The role of the AnxA2 gene during virus infection has not yet been reported. In this study, we observed that AnxA2 gene expression was up-regulated in BHK-21 cells infected with the virus. Additionally, overexpression of the AnxA2 gene promoted the release of mature virus particles, whereas BEFV replication was remarkably inhibited after reducing AnxA2 gene expression by using the small interfering RNA (siRNA). For viral proteins, overexpression of the Matrix (M) gene promotes the release of mature virus particles. Moreover, the AnxA2 protein interaction with the M protein of BEFV was confirmed by GST pull-down and co-immunoprecipitation assays. Experimental results indicate that the C-terminal domain (268–334 aa) of AxnA2 contributes to this interaction. An additional mechanistic study showed that AnxA2 protein interacts with M protein and mediates the localization of the M protein at the plasma membrane. Furthermore, the absence of the AnxA2-V domain could attenuate the effect of AnxA2 on BEFV replication. These findings can contribute to elucidating the regulation of BEFV replication and may have implications for antiviral strategy development.
Go to : 
References
1. Walker PJ, Klement E. Epidemiology and control of bovine ephemeral fever. Vet Res (Faisalabad). 2015; 46:124.

2. Hayama Y, Moriguchi S, Yanase T, Suzuki M, Niwa T, Ikemiyagi K, Nitta Y, Yamamoto T, Kobayashi S, Murai K, Tsutsui T. Epidemiological analysis of bovine ephemeral fever in 2012-2013 in the subtropical islands of Japan. BMC Vet Res. 2016; 12:47.

3. Du X, Zhou J. Application of biosensors to detection of epidemic diseases in animals. Res Vet Sci. 2018; 118:444–448.

4. Hou P, Zhao G, He C, Wang H, He H. Biopanning of polypeptides binding to bovine ephemeral fever virus G1 protein from phage display peptide library. BMC Vet Res. 2018; 14:3.

5. He CQ, Liu YX, Wang HM, Hou PL, He HB, Ding NZ. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet Microbiol. 2016; 182:50–56.

6. Jayakar HR, Jeetendra E, Whitt MA. Rhabdovirus assembly and budding. Virus Res. 2004; 106:117–132.

7. Zan J, Liu J, Zhou JW, Wang HL, Mo KK, Yan Y, Xu YB, Liao M, Su S, Hu RL, Zhou JY. Rabies virus matrix protein induces apoptosis by targeting mitochondria. Exp Cell Res. 2016; 347:83–94.

8. Jayakar HR, Murti KG, Whitt MA. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J Virol. 2000; 74:9818–9827.

10. Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D. Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med. 2016; 14:89.

11. Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994; 1197:63–93.

12. Aliyu IA, Ling KH, Md Hashim N, Chee HY. Annexin A2 extracellular translocation and virus interaction: A potential target for antivirus-drug discovery. Rev Med Virol. 2019; 29:e2038.

13. Solbak SM, Abdurakhmanov E, Vedeler A, Danielson UH. Characterization of interactions between hepatitis C virus NS5B polymerase, annexin A2 and RNA – effects on NS5B catalysis and allosteric inhibition. Virol J. 2017; 14:236.

14. Woodham AW, Taylor JR, Jimenez AI, Skeate JG, Schmidt T, Brand HE, Da Silva DM, Kast WM. Small molecule inhibitors of the annexin A2 heterotetramer prevent human papillomavirus type 16 infection. J Antimicrob Chemother. 2015; 70:1686–1690.

15. Wang J, Song J, Clark G, Roux SJ. ANN1 and ANN2 function in post-phloem sugar transport in root tips to affect primary root growth. Plant Physiol. 2018; 178:390–401.

16. Luo S, Xie C, Wu P, He J, Tang Y, Xu J, Zhao S. Annexin A2 is an independent prognostic biomarker for evaluating the malignant progression of laryngeal cancer. Exp Ther Med. 2017; 14:6113–6118.

17. Shaker MK, Abdel Fattah HI, Sabbour GS, Montasser IF, Abdelhakam SM, El Hadidy E, Yousry R, El Dorry AK. Annexin A2 as a biomarker for hepatocellular carcinoma in Egyptian patients. World J Hepatol. 2017; 9:469–476.

19. Nazmi AR, Ozorowski G, Pejic M, Whitelegge JP, Gerke V, Luecke H. N-terminal acetylation of annexin A2 is required for S100A10 binding. Biol Chem. 2012; 393:1141–1150.

20. Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, Isas JM, Langen R, Kast WM. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One. 2012; 7:e43519.

21. Grindheim AK, Saraste J, Vedeler A. Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim Biophys Acta, Gen Subj. 2017; 1861:2515–2529.

22. Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM. Annexin A2 is a novel RNA-binding protein. J Biol Chem. 2004; 279:8723–8731.

23. Mickleburgh I, Burtle B, Hollås H, Campbell G, Chrzanowska-Lightowlers Z, Vedeler A, Hesketh J. Annexin A2 binds to the localization signal in the 3′untranslated region of c-myc mRNA. FEBS J. 2005; 272:413–421.

24. Wright JF, Kurosky A, Wasi S. An endothelial cell-surface form of annexin II binds human cytomegalovirus. Biochem Biophys Res Commun. 1994; 198:983–989.

25. Wright JF, Kurosky A, Pryzdial EL, Wasi S. Host cellular annexin II is associated with cytomegalovirus particles isolated from cultured human fibroblasts. J Virol. 1995; 69:4784–4791.

26. Backes P, Quinkert D, Reiss S, Binder M, Zayas M, Rescher U, Gerke V, Bartenschlager R, Lohmann V. Role of annexin A2 in the production of infectious hepatitis C virus particles. J Virol. 2010; 84:5775–5789.

27. LeBouder F, Morello E, Rimmelzwaan GF, Bosse F, Péchoux C, Delmas B, Riteau B. Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin. J Virol. 2008; 82:6820–6828.

28. Ma Y, Sun J, Gu L, Bao H, Zhao Y, Shi L, Yao W, Tian G, Wang X, Chen H. Annexin A2 (ANXA2) interacts with nonstructural protein 1 and promotes the replication of highly pathogenic H5N1 avian influenza virus. BMC Microbiol. 2017; 17:191.

29. Liu Y, Gao P. Modulation of hepatitis B surface antigen secretion by annexin II expressed in hepatitis B virus-producing hepatoma cells. Mol Med Rep. 2014; 10:3113–3117.

30. Sheng C, Liu X, Jiang Q, Xu B, Zhou C, Wang Y, Chen J, Xiao M. Annexin A2 is involved in the production of classical swine fever virus infectious particles. J Gen Virol. 2015; 96:1027–1032.

31. Hou P, Wang H, Zhao G, He C, He H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. 2017; 13:386.

32. Wang X, Ju Z, Huang J, Hou M, Zhou L, Qi C, Zhang Y, Gao Q, Pan Q, Li G, Zhong J, Wang C. The relationship between the variants of the bovine MBL2 gene and milk production traits, mastitis, serum MBL-C levels and complement activity. Vet Immunol Immunopathol. 2012; 148:311–319.

33. Chang XB, Yang YQ, Gao JC, Zhao K, Guo JC, Ye C, Jiang CG, Tian ZJ, Cai XH, Tong GZ, An TQ. Annexin A2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication. Vet Res (Faisalabad). 2018; 49:75.

34. Wang XG, Huang JM, Feng MY, Ju ZH, Wang CF, Yang GW, Yuan JD, Zhong JF. Regulatory mutations in the A2M gene are involved in the mastitis susceptibility in dairy cows. Anim Genet. 2014; 45:28–37.
35. Li S, van Os GM, Ren S, Yu D, Ketelaar T, Emons AM, Liu CM. Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 2010; 154:1819–1830.
36. Lv L, Zhao G, Wang H, He H. Cholesterol 25-Hydroxylase inhibits bovine parainfluenza virus type 3 replication through enzyme activity-dependent and -independent ways. Vet Microbiol. 2019; 239:108456.

37. Hou P, Zhao M, He W, He H, Wang H. Cellular microRNA bta-miR-2361 inhibits bovine herpesvirus 1 replication by directly targeting EGR1 gene. Vet Microbiol. 2019; 233:174–183.

38. Ma W, Wang H, He H. Bovine herpesvirus 1 tegument protein UL41 suppresses antiviral innate immune response via directly targeting STAT1. Vet Microbiol. 2019; 239:108494.

39. Liang J, Sagum CA, Bedford MT, Sidhu SS, Sudol M, Han Z, Harty RN. Chaperone-mediated autophagy protein BAG3 negatively regulates ebola and marburg VP40-mediated egress. PLoS Pathog. 2017; 13:e1006132.

40. Koga R, Kubota M, Hashiguchi T, Yanagi Y, Ohno S. Annexin A2 mediates the localization of measles virus matrix protein at the plasma membrane. J Virol. 2018; 92:92.

41. Dziduszko A, Ozbun MA. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J Virol. 2013; 87:7502–7515.

42. Yang SL, Chou YT, Wu CN, Ho MS. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol. 2011; 85:11809–11820.

43. Li J, Guo D, Huang L, Yin M, Liu Q, Wang Y, Yang C, Liu Y, Zhang L, Tian Z, Cai X, Yu L, Weng C. The interaction between host Annexin A2 and viral Nsp9 is beneficial for replication of porcine reproductive and respiratory syndrome virus. Virus Res. 2014; 189:106–113.

44. Kwak H, Park MW, Jeong S. Annexin A2 binds RNA and reduces the frameshifting efficiency of infectious bronchitis virus. PLoS One. 2011; 6:e24067.

45. Lai CK, Jeng KS, Machida K, Lai MM. Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J Virol. 2008; 82:8838–8848.

46. Saxena V, Lai CK, Chao TC, Jeng KS, Lai MM. Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft. J Virol. 2012; 86:4139–4150.

47. Soh TK, Whelan SP. Tracking the fate of genetically distinct vesicular stomatitis virus matrix proteins highlights the role for late domains in assembly. J Virol. 2015; 89:11750–11760.

48. Hollås H, Aukrust I, Grimmer S, Strand E, Flatmark T, Vedeler A. Annexin A2 recognises a specific region in the 3′-UTR of its cognate messenger RNA. Biochim Biophys Acta. 2006; 1763:1325–1334.

49. Santos-Valencia JC, Cancio-Lonches C, Trujillo-Uscanga A, Alvarado-Hernández B, Lagunes-Guillén A, Gutiérrez-Escolano AL. Annexin A2 associates to feline calicivirus RNA in the replication complexes from infected cells and participates in an efficient viral replication. Virus Res. 2019; 261:1–8.

Go to : 
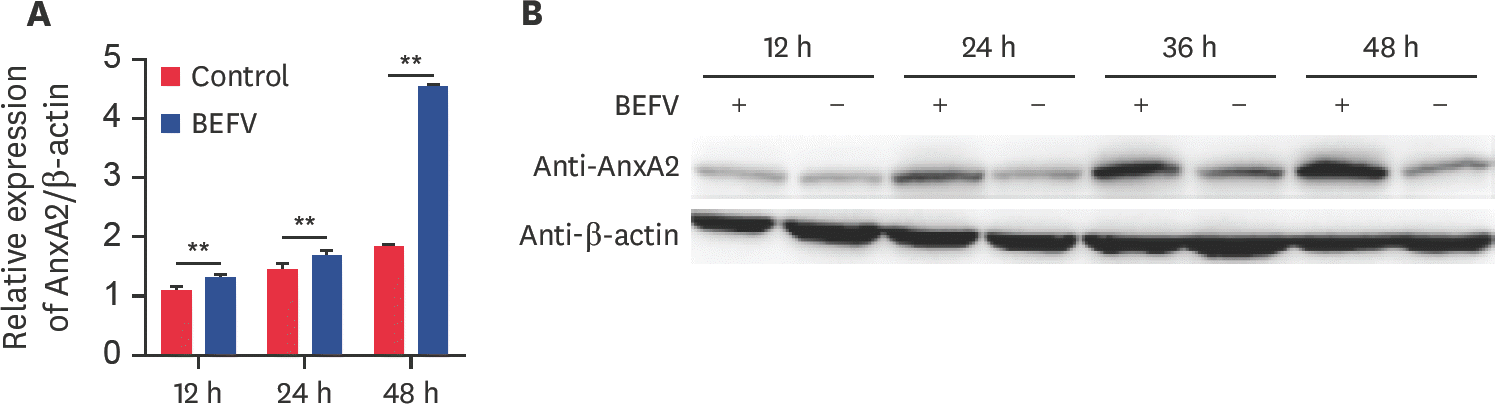 | Fig. 1.BEFV infection up-regulates AnxA2 gene expression in BHK-21 cells. (A) AnxA2 gene expression levels in BHK-21 cells infected with BEFV at an MOI of 0.1 at 12, 24, and 48 hpi were determined by performing real-time qPCR with β-actin used as the standard. Data indicate the relative fold-change from the control cells. The average values ± SD were determined from three independent experiments. (B) AnxA2 protein expression in BHK-21 cells mock-infected or infected with BEFV at an MOI of 0.1 at 12, 24, 36, and 48 hpi determined by western blotting with anti-AnxA2 antibody and β-actin used as the control. AnxA2, annexin A2; BEFV, bovine ephemeral fever virus; MOI, multiplicity of infection; hpi, hours post infection; qPCR, quantitative polymerase chain reaction. ** p < 0.01. |
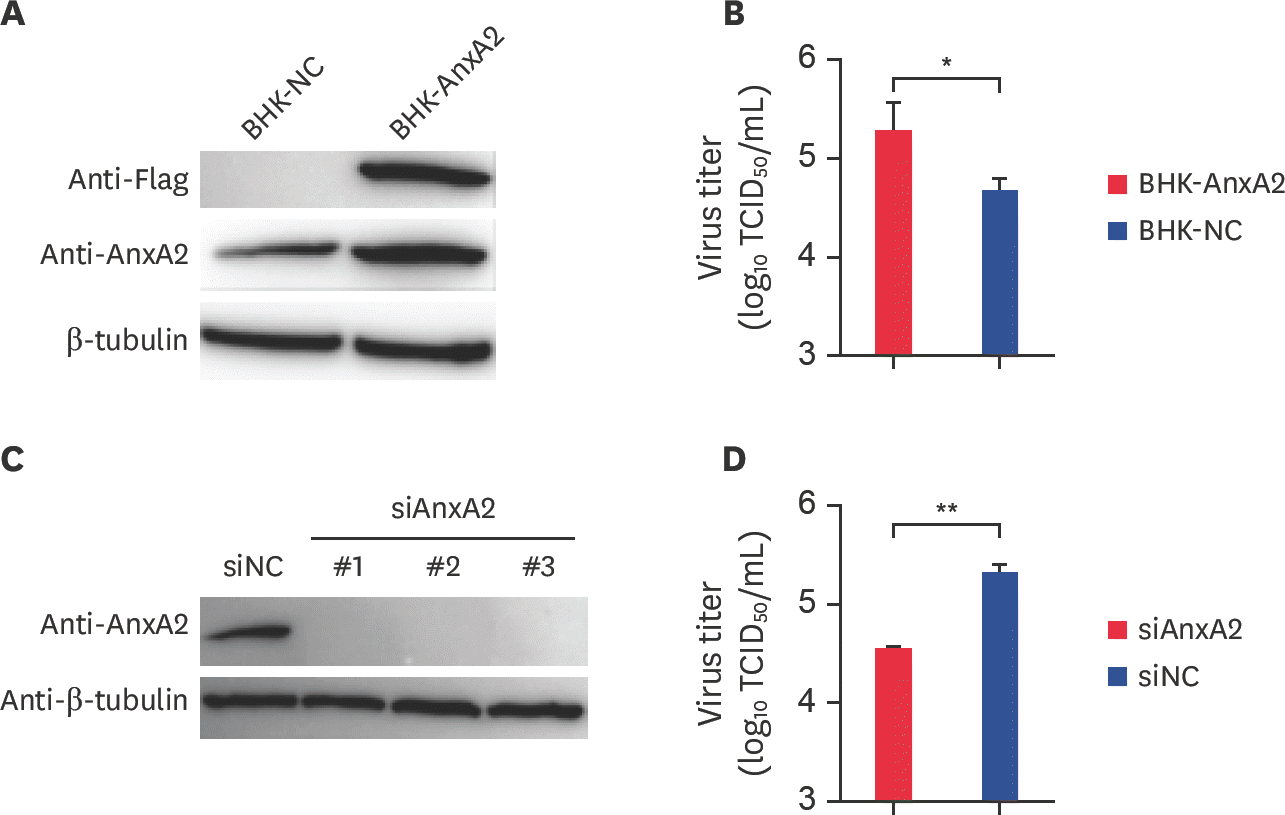 | Fig. 2.AnxA2 gene expression promotes BEFV replication in BHK-21 cells. (A) The expression levels of AnxA2 protein in a stable AnxA2 overexpression cell line (BHK-AnxA2) and the negative control cell line (BHK-NC) were assayed by western blotting with anti-AnxA2 and anti-Flag antibody; β-actin was used as the control. (B) BHK-AnxA2 and BHK-NC cells were inoculated with BEFV at an MOI of 0.1 for 48 h, and the resulting titers in the supernatant of BEFV-infected cells were determined by TCID50 assay. The mean and standard deviation values were determined from three independent experiments. (C) The knock-down efficiency of AnxA2-siRNA in BHK-21 cell lines was examined by western blot analysis with anti-AnxA2 antibody and control siRNA with β-actin as the internal control. (D) SiAnxA2 and siNC were infected with BEFV at an MOI of 0.1 for 48 h and the release of progeny virus particles in cell supernatants was assessed. The data are average and SD values obtained from three independent experiments. AnxA2, annexin A2; BEFV, bovine ephemeral fever virus; MOI, multiplicity of infection; siRNA, small interfering RNA; siNC, scrambled siRNA for AnxA2; siAnxA2, effective siRNA-AnxA2 targeting the AnxA2 gene; NC, negative control; TCID50, median tissue culture infective dose. * p < 0.05; ** p < 0.01. |
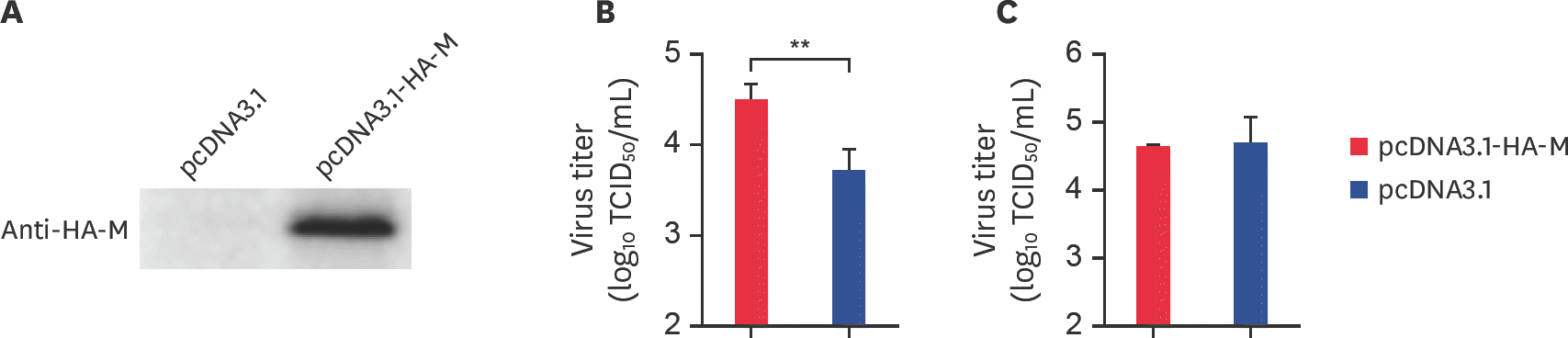 | Fig. 3.The M gene promotes BEFV replication in BHK-21 cells. (A) pcDNA3.1-HA-M and the vector control were transfected separately into BHK-21 cells. At 24 hpt, the expression of M protein was assayed by western blotting with rabbit anti-HA. (B) BHK-21 cells were transfected with pcDNA3.1-HA-M and the vector control at 24 hpt and infected with BEFV at an MOI of 0.1. After 48 hpi, cell supernatants were collected and assayed by determining TCID50. (C) Cell precipitates were collected and assayed by determiningTCID50. M, matrix; BEFV, bovine ephemeral fever virus; MOI, multiplicity of infection; hpi, hours post infection; TCID50, median tissue culture infective dose. ** p < 0.01. |
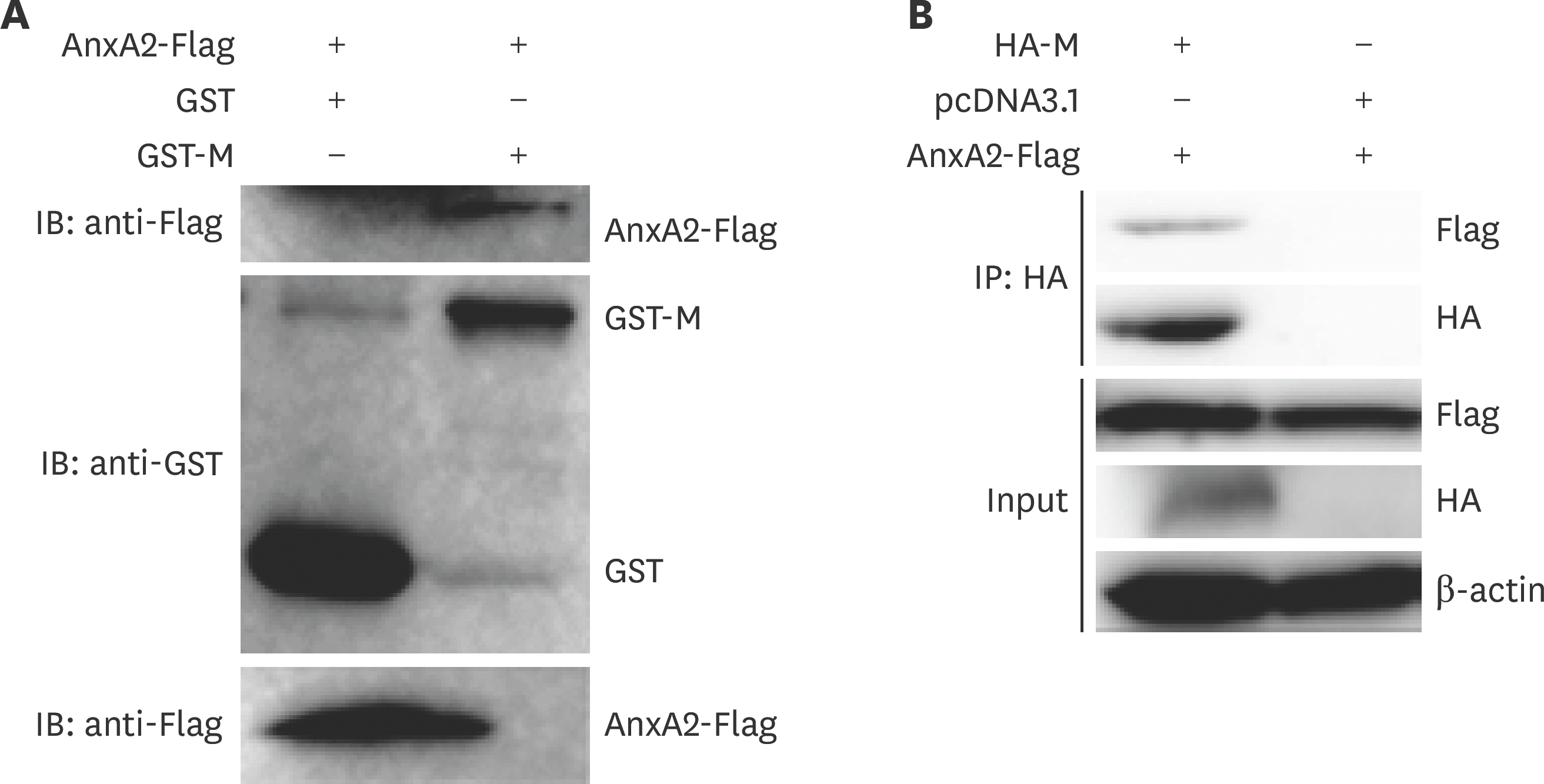 | Fig. 4.AnxA2 interacts with bovine ephemeral fever virus M protein in BHK-21 cells. (A) AnxA2 interaction with M assessed by GST pull-down assay. GST or GST-M fusion protein was combined with BHK-21 cell lysates expressing pcDNA3.1-AnxA2-Flag. GST and GST-M were combined with Beaver Beads (GSH). After washing, proteins were eluted from the beads and detected by performing sodium dodecyl sulfate polyacrylamide gel electrophoresis. The presence of AnxA2 was tested by immune-blotting with rabbit anti-Flag. The expression of GST and GST-M was determined by immunoblotting with rabbit anti-GST. (B) The interaction between AnxA2 and M proteins was identified by performing co-immunoprecipitation assays. BHK-21 cells were co-transfected with pcDNA3.1-AnxA2-Flag and pcDNA3.1-HA-M. At 24 hpt, whole-cell lysates were immunoprecipitated with the anti-HA antibody. Subsequently, the complex underwent western blot analysis using specific antibodies (anti-HA and anti-Flag) as indicated. AnxA2, annexin A2; M, matrix. |
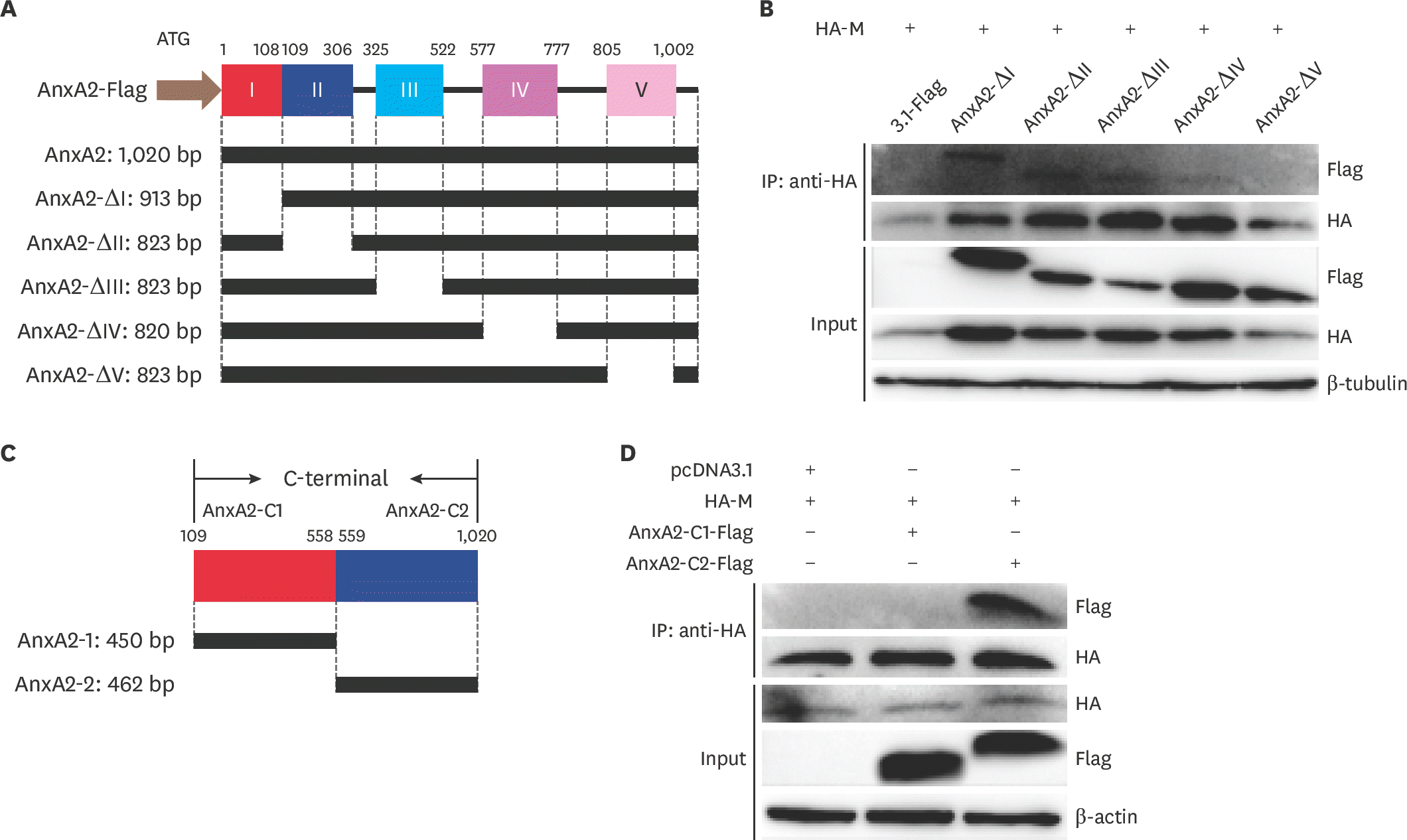 | Fig. 5.Identification of the binding domains of AnxA2 that interact with M in BHK-21 cells. (A) A schematic map for predicting the domains of AnxA2 protein. The full-length AnxA2 protein and five truncated AnxA2 mutants were examined. (B) Analysis of the interaction between AnxA2 and M proteins by immunoprecipitation analysis. BHK-21 cells were transfected with the pcDNA3.1-Flag vector containing AnxA2 (WT or mutant) and pcDNA3.1-HA-M. At 24 hpt, the cell supernatant was co-immunoprecipitated using anti-HA and anti-Flag antibodies. Co-immunoprecipitation of the AnxA2–M interaction was tested by western blot analysis with anti-Flag and anti-HA antibodies. Cell lysates were separated by performing 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and assessed by western blotting with anti-Flag and anti-HA antibodies. (C) Two truncated AnxA2 C-terminal mutants were constructed. (D) BHK-21 cells were transfected with pcDNA3.1-HA-M and AnxA2 (WT or mutant) or pcDNA3.1-Flag. Co-immunoprecipitation results for the AnxA2–M interaction were examined by western blot analysis with the anti-Flag antibody. AnxA2, annexin A2; M, matrix; WT, wild type; hpt, hours post transfection. |
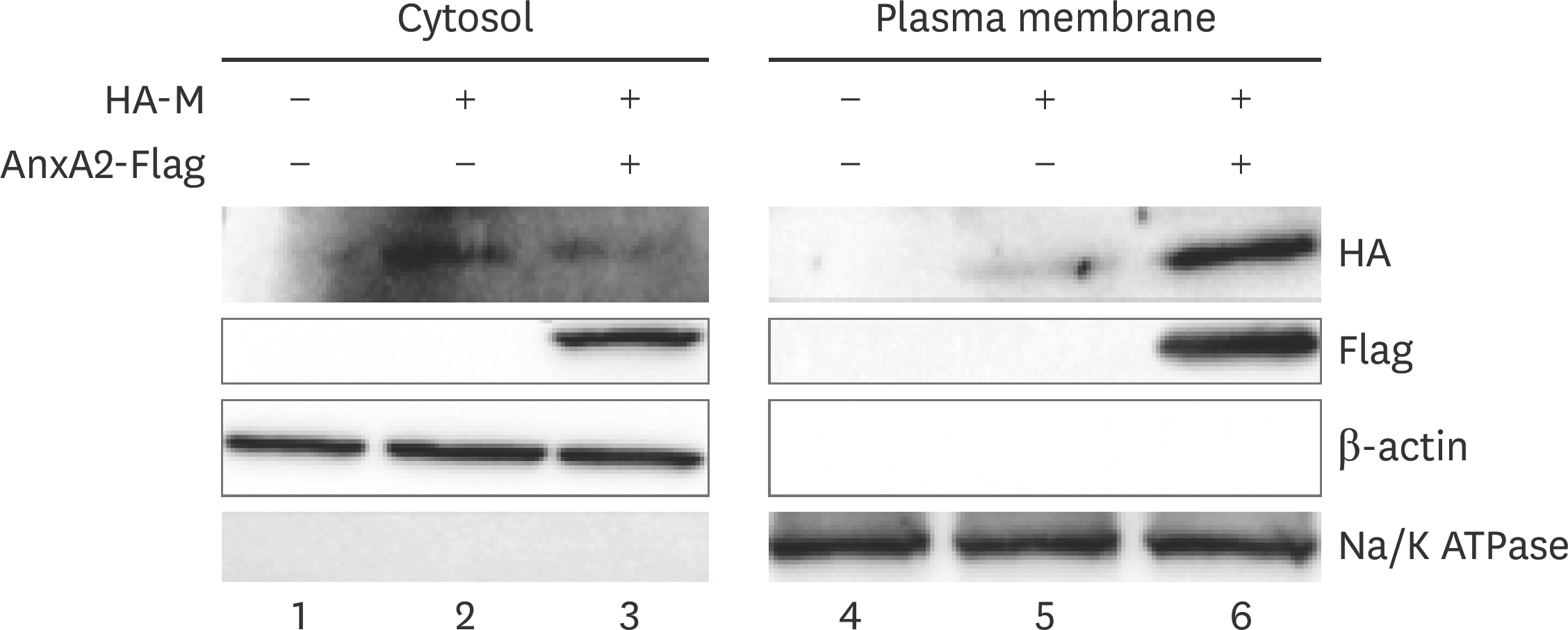 | Fig. 6.AnxA2 expression increases plasma membrane localization of M protein in BHK-21 cells. BHK-21 cells were transfected with vector (pcDNA3.1) alone, M + vector, or M + AnxA2 and at 24 hpt cells were collected, PM components separated, and western blotting performed. Na/K ATPase (Abways, China) was used as a control protein for the PM components, and β-actin (Abways) was used as a control for the cytosol fraction. AnxA2, annexin A2; M, matrix; hpt, hours post transfection; PM, plasma membrane. |
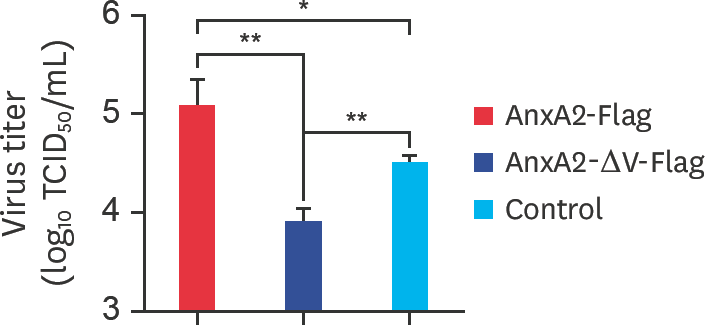 | Fig. 7.The V domain of the AnxA2 gene reduces the generation of BEFV progeny virus particles in BHK-21 cells. BHK-21 cells were transfected with vector control, AnxA2 + vector, or AnxA2-ΔV + vector, and then infected with the virus at a multiplicity of infection of 0.1 for 48 h. The supernatant titers of the BEFV-infected cells were determined by performing TCID50 assays. AnxA2, annexin A2; BEFV, bovine ephemeral fever virus; TCID50, median tissue culture infective dose. * p < 0.05; ** p < 0.01. |
Table 1.
List of primers used to create AnxA2-WT and AnxA2 deletion mutants
Table 2.
List of primers used during knock-down of the AnxA2 gene




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download