Abstract
In this study, whiteleg shrimp (Penaeus vannamei) imported from Vietnam were collected from South Korean markets, and examined for 2 viruses: infectious hypodermal and hematopoietic necrosis virus (IHHNV, recently classified as decapod penstyldensovirus-1), and white spot syndrome virus (WSSV). Among 58 samples, we detected IHHNV in 23 samples and WSSV in 2 samples, using polymerase chain reaction and sequencing analyses. This is the first report of IHHNV and WSSV detection in imported shrimp, suggesting that greater awareness and stricter quarantine policies regarding viruses infecting shrimp imported to South Korea are required.
References
1. Food and Agriculture Organization of the United Nations. FishStatJ—Software for Fishery Statistical Time Series [Internet]. Food and Agriculture Organization of the United Nations;2016. [updated 2016 July 21; cited 2019 October 1]. Available from:. http://www.fao.org/fishery/statistics/software/fishstatj/en.
2. Dastidar PG, Mallik A, Mandal N. Contribution of shrimp diseases research to the development of the shrimp aquaculture industry: an analysis of the research and innovation structure across the countries. Scientometrics. 2013; 97:659–674.
3. Wyban J. World shrimp farming revolution: industry impact of domestication, breeding and widespread use of specific pathogen free Penaeus vannamei. Browdy CL, Jory DE, editors. (eds.).The Rising Tide: Proceedings of the Special Session on Sustainable Shrimp Farming. pp.p. 12–21. World Aquaculture;Baton Rouge: 2009.
4. Walker PJ, Mohan CV. Viral disease emergence in shrimp aquaculture: origins, impact and the effectiveness of health management strategies. Aquaculture. 2009; 1:125–154.

5. King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press;San Diego: 2012.
6. Tang KF, Lightner DV. Infectious hypodermal and hematopoietic necrosis virus (IHHNV)-related sequences in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Res. 2006; 118:185–191.
7. Nunan LM, Lightner DV. Optimized PCR assay for detection of white spot syndrome virus (WSSV). J Virol Methods. 2011; 171:318–321.

8. National Fishery Products Quality Management Service. Quarantine of Imported and Exported Aquatic Organisms [Internet]. National Fishery Products Quality Management Service;2019. [updated 2019;cited 2019 October 1]. Available from:. http://www.nfqs.go.kr/foreign/en/sub1_1.html.
9. Kim JH, Choresca CH Jr, Shin SP, Han JE, Jun JW, Han SY, Park SC. Detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei shrimp cultured in South Korea. Aquaculture. 2011; 313:161–164.
10. Wang Q, White LB, Redman RM, Lightner DV. Per os challenge of Litopenaeus vannamei postlarvae and Farfantepenaeus duorarum juveniles with six geographic isolates of white spot syndrome virus. Aquaculture. 1999; 170:179–194.
11. Park JH, Lee YS, Lee S, Lee Y. An infectious viral disease of penaeid shrimp newly found in Korea. Dis Aquat Organ. 1998; 34:71–75.

12. Yeh SP, Chen YN, Hsieh SL, Cheng W, Liu CH. Immune response of white shrimp, Litopenaeus vannamei, after a concurrent infection with white spot syndrome virus and infectious hypodermal and hematopoietic necrosis virus. Fish Shellfish Immunol. 2009; 26:582–588.
13. Han JE, Kim JE, Jo HY, Eun JS, Lee CR, Kim JH, Lee KJ, Kim JW. Increased susceptibility of white spot syndrome virus-exposed Penaeus vannamei to Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease. Aquaculture. 2019; 512:734333.
14. De Silva BC, Hossain S, Wimalasena SH, Pathirana HN, Heo GJ. Putative virulence traits and antibiogram profile of Aeromonas spp. isolated from frozen white-leg shrimp (Litopenaeus vannamei) marketed in Korea. J Food Saf. 2018; 38:e12470.
15. Lightner DV, Redman RM, Poulos BT, Nunan LM, Mari JL, Hasson KW. Risk of spread of penaeid shrimp viruses in the Americas by the international movement of live and frozen shrimp. Rev Sci Tech. 1997; 16:146–160.
Fig. 1.
Phylogenetic analyses of the representative IHHNV (A) and WSSV (B) detected in this study. Maximum-likelihood trees were reconstructed based on the obtained sequences of the IHHNV-positive sample (sample No. 19-005-h5 corresponding to the strain IHHNV-KK-VIET1, MN481525 [390 bp]) and WSSV-positive sample (sample No. 19-005-h5 corresponding to the strain WSSV-KK-VIET1, MN481520 [936 bp]), respectively. Tiger shrimp, Penaeus monodon (AY124937.1), and Jamaican Bromeliad Crab (Metopaulias depressus) WSSV-like virus (KR820241.1) were used as outgroup for IHHNV and WSSV phylogeny, respectively. Numbers at the branches indicate bootstrap values obtained with 1,000 replicates. The scale bar represents 0.005 and 0.1 nucleotide substitutions per site, respectively. IHHNV, infectious hypodermal and hematopoietic necrosis virus; WSSV, white spot syndrome virus.
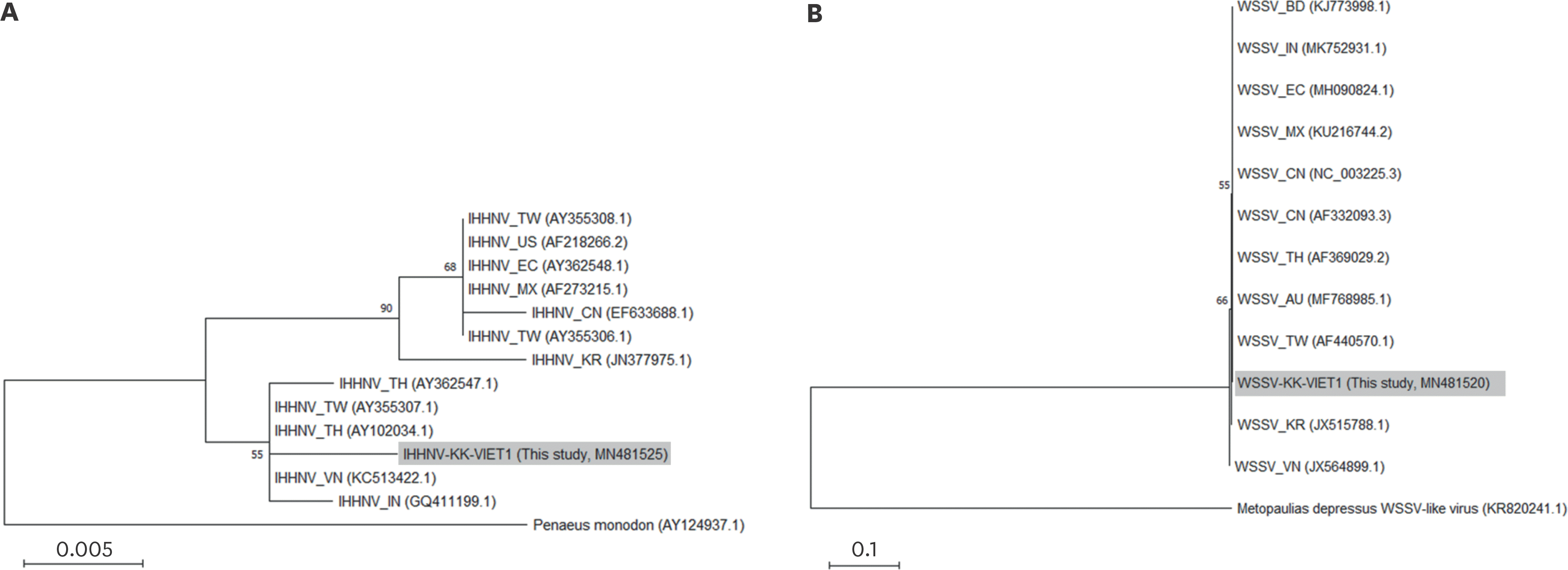
Table 1.
Shrimp samples and PCR results from this study
| Samples | Viral nucleic acids detected by PCR assay | Samples | Viral nucleic acids detected by PCR assay |
|---|---|---|---|
| 19-005-a5 | –* | 19-006-1 | IHHNV |
| 19-005-b5 | IHHNV | 19-006-2 | – |
| 19-005-c5 | – | 19-006-3 | – |
| 19-005-d5 | – | 19-006-4 | IHHNV |
| 19-005-e5 | IHHNV | 19-006-5 | IHHNV |
| 19-005-f5 | – | 19-006-6 | – |
| 19-005-g5 | IHHNV | 19-006-7 | IHHNV |
| 19-005-h5 | IHHNV + WSSV† | 19-006-8 | IHHNV |
| 19-005-B5 | IHHNV | 19-006-9 | IHHNV |
| 19-005-C5 | – | 19-006-10 | – |
| 19-005-D4 | – | 19-006-11 | – |
| 19-005-E5 | IHHNV | 19-006-12 | – |
| 19-005-G5 | IHHNV | 19-006-13 | – |
| 19-005-H5 | IHHNV | 19-006-14 | – |
| 19-004-A1 | – | 19-006-15 | – |
| 19-004-B1 | – | 19-006-16 | – |
| 19-004-C1 | – | 19-006-17 | – |
| 19-004-D1 | IHHNV | 19-006-18 | – |
| 19-004-E1 | – | 19-006-19 | – |
| 19-004-F1 | – | 19-006-20 | IHHNV |
| 19-004-G1 | – | 19-006-21 | – |
| 19-004-H1 | – | 19-006-22 | IHHNV + WSSV |
| 19-004-I1 | – | 19-006-23 | IHHNV |
| 19-004-J1 | IHHNV | 19-006-24 | – |
| 19-004-A2 | – | 19-006-25 | – |
| 19-004-D2 | – | 19-006-26 | – |
| 19-004-F2 | IHHNV | 19-006-27 | IHHNV |
| 19-004-D3 | – | 19-006-28 | IHHNV |
| 19-004-F3 | – | 19-006-29 | IHHNV |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download