Abstract
Objective
This prospective randomized controlled pilot study aimed to find whether gelatin-thrombin matrix used as a tissue sealant (FloSeal) can prevent the occurrence of pelvic lymphocele in patients with gynecologic cancer who has undergone pelvic lymphadenectomy.
Methods
Each patient, who undergo a laparotomic pelvic lymph node dissection on both sides, was randomly assigned for FloSeal application on 1 side of the pelvis. The other side of the pelvis without any product application being the control side. The amount of lymph drainage at each side of the pelvis was measured for 3 days, and computed tomography scans were obtained 7 days and 6 months after surgery for detection of pelvic lymphocele.
Results
Among 37 cases, the median amount of lymph drainage was significantly decreased in the hemi-pelvis treated with FloSeal compared to the control hemi-pelvis (p=0.025). The occurrence of lymphocele was considerably reduced in treated hemi-pelvis (8/37, 21.6%) compared with control hemi-pelvis (12/37, 32.4%) after 7 post-operative days (p=0.219), and more decreased in the treated hemi-pelvis (5/37, 13.5%) compared with control hemi-pelvis (9/37, 24.3%) after postoperative 6 months (p=0.344).
References
1. Selman TJ, Mann C, Zamora J, Appleyard TL, Khan K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: a systematic review and meta-analysis. CMAJ. 2008; 178:855–62.

2. Selman TJ, Mann CH, Zamora J, Khan KS. A systematic review of tests for lymph node status in primary endometrial cancer. BMC Womens Health. 2008; 8:8.

3. Petru E, Tamussino K, Lahousen M, Winter R, Pickel H, Haas J. Pelvic and paraaortic lymphocysts after radical surgery because of cervical and ovarian cancer. Am J Obstet Gynecol. 1989; 161:937–41.

4. Logmans A, Kruyt RH, de Bruin HG, Cox PH, Pillay M, Trimbos JB. Lymphedema and lymphocysts following lymphadenectomy may be prevented by omentoplasty: a pilot study. Gynecol Oncol. 1999; 75:323–7.

5. Suzuki M, Ohwada M, Sato I. Pelvic lymphocysts following retroperitoneal lymphadenectomy: retroperitoneal partial “no-closure” for ovarian and endometrial cancers. J Surg Oncol. 1998; 68:149–52.

6. Benedetti-Panici P, Maneschi F, Cutillo G, D'Andrea G, di Palumbo VS, Conte M, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecol Oncol. 1997; 65:478–82.

7. Yamamoto R, Saitoh T, Kusaka T, Todo Y, Takeda M, Okamoto K, et al. Prevention of lymphocyst formation following systematic lymphadenectomy. Jpn J Clin Oncol. 2000; 30:397–400.

8. Nezhat F, Yadav J, Rahaman J, Gretz H 3rd, Gardner GJ, Cohen CJ. Laparoscopic lymphadenectomy for gynecologic malignancies using ultrasonically activated shears: analysis of first 100 cases. Gynecol Oncol 2005;97:813–9.

9. Gallotta V, Fanfani F, Rossitto C, Vizzielli G, Testa A, Scambia G, et al. A randomized study comparing the use of the Ligaclip with bipolar energy to prevent lymphocele during laparoscopic pelvic lymphadenectomy for gynecologic cancer. Am J Obstet Gynecol. 2010; 203:483. e1–6.

10. Scholz HS, Petru E, Benedicic C, Haas J, Tamussino K, Winter R. Fibrin application for preventing lymphocysts after retroperitoneal lymphadenectomy in patients with gynecologic malignancies. Gynecol Oncol. 2002; 84:43–6.
11. Tinelli A, Giorda G, Manca C, Pellegrino M, Prudenzano R, Guido M, et al. Prevention of lymphocele in female pelvic lymphadenectomy by a collagen patch coated with the human coagulation factors: a pilot study. J Surg Oncol. 2012; 105:835–40.

12. Waldert M, Remzi M, Klatte T, Klingler HC. FloSeal reduces the incidence of lymphoceles after lymphadenectomies in laparoscopic and robot-assisted extraperitoneal radical prostatectomy. J Endourol. 2011; 25:969–73.

13. Köhler C, Kyeyamwa S, Marnitz S, Tsunoda A, Vercelino F, Schneider A, et al. Prevention of lymphoceles using FloSeal and CoSeal after laparoscopic lymphadenectomy in patients with gynecologic malignancies. J Minim Invasive Gynecol. 2015; 22:451–5.

Fig. 1.
Flow chart of participating patients presented by the Consolidated Standards of Reporting Trials diagram.
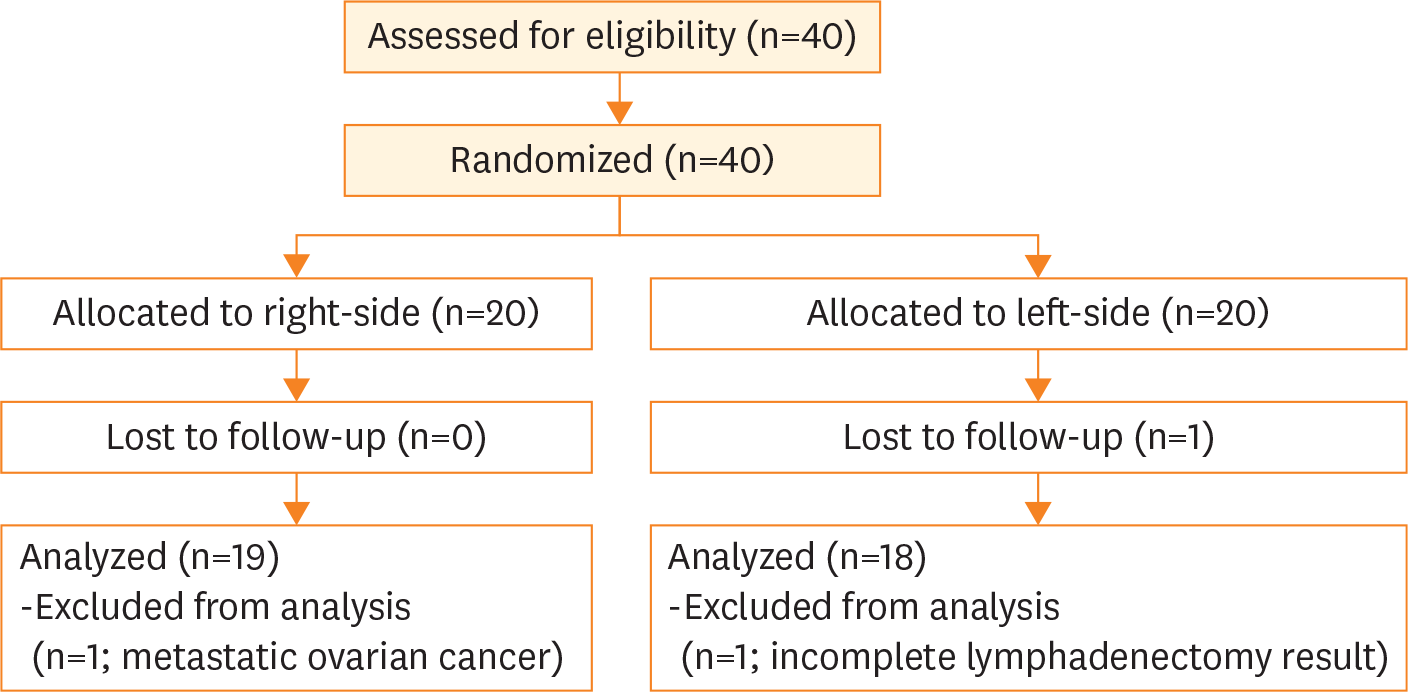
Table 1.
Patients characteristics between the 2 groups according to the site of FloSeal application (n=37)
| Characteristics | Right side (n=19) | Left side (n=18) | p-value |
|---|---|---|---|
| Age | 52 (34–70) | 52 (27–67) | 0.915* |
| Type of tumor | 0.980† | ||
| Cervical cancer | 5 (26.3) | 5 (27.8) | |
| Endometrial cancer | 6 (31.6) | 6 (33.3) | |
| Ovarian cancer | 8 (42.1) | 7 (38.9) | |
| Adjuvant irradiation | 4 (21.1) | 5 (27.8) | 0.634† |
| Concurrent PALN dissection | 19 (100.0) | 16 (88.9) | 0.230† |
| No. of PLN | 19 (6–35) | 19 (5–42) | 0.456* |
| No. of PALN | 6 (1–20) | 6 (1–25) | 0.336* |
| Blood loss (mL) | 700 (50–4,000) | 500 (150–1,600) | 0.461* |
Table 2.
Total amount of 3-day pelvic lymph drainage and incidence of lymphocele at each hemi-pelvis at 7 days and 6 months postoperative
| Characteristics | Control hemi-pelvis (n=37) | Study hemi-pelvis (n=37) | p-value |
|---|---|---|---|
| Amount of lymph drainage, mL | 620 (102–1,390) | 400 (88–1,320) | 0.025* |
| Lymphocele at post-operative | |||
| 1 wk | 12 (32.4) | 8 (21.6) | 0.219† |
| 6 mo | 9 (24.3) | 5 (13.5) | 0.344† |
Table 3.
Distribution and size of pelvic lymphocele at 6 months after operation
| Location | Control hemi-pelvis (n=37) | Study hemi-pelvis (n=37) |
|---|---|---|
| External iliac | 9 (2; 1–4) | 4 (2; 1.0–2.5) |
| Internal iliac | 0 | 0 |
| Common iliac | 0 | 1 (4; 4–4) |
Table 4.
Preventive methods against lymphocele after pelvic lymphadenectomy in gynecologic malignancies




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download