Abstract
Most patients with hepatocellular carcinoma (HCC) are diagnosed at an advanced stage of disease. Until recently, systemic treatment options that showed survival benefits in HCC have been limited to tyrosine kinase inhibitors, antibodies targeting oncogenic signaling pathways or VEGF receptors. The HCC tumor microenvironment is characterized by a dysfunction of the immune system through multiple mechanisms, including accumulation of various immunosuppressive factors, recruitment of regulatory T cells and myeloid-derived suppressor cells, and induction of T cell exhaustion accompanied with the interaction between immune checkpoint ligands and receptors. Immune checkpoint inhibitors (ICIs) have been interfered this interaction and have altered therapeutic landscape of multiple cancer types including HCC. In this review, we discuss the use of anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies in the treatment of advanced HCC. However, ICIs as a single agent do not benefit a significant portion of patients. Therefore, various clinical trials are exploring possible synergistic effects of combinations of different ICIs (anti-PD-1/PD-L1 and anti-CTLA-4 antibodies) or ICIs and target agents. Combinations of ICIs with locoregional therapies may also improve therapeutic responses.
Go to : 
References
1. Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017; 3:1683–1691.
3. Lee JS, Cho IR, Lee HW, Jeon MY, Lim TS, Baatarkhuu O, Kim DY, Han KH, Park JY. Conditional survival estimates improve over time for patients with hepatocellular carcinoma: an analysis for Nationwide Korea Cancer Registry Database. Cancer Res Treat. 2019; 51:1347–1356.

4. Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010; 28:3994–4005.

5. European Association for the Study of the Liver. Management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.
6. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

7. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.

8. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66.

9. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018; 379:54–63.

10. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20:282–296.

11. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011; 331:1565–1570.

12. Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin Cancer Res. 2018; 24:1518–1524.

14. Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010; 31:1409–1420.

15. Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019; 38:396.

16. Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: implications for prognosis and therapeutic applications. Liver Int. 2019; 39:1608–1621.

17. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015; 12:681–700.

18. Chon YE, Park H, Hyun HK, Ha Y, Kim MN, Kim BK, Lee JH, Kim SU, Kim DY, Ahn SH, et al. Development of a new nomogram including neutrophil-to-lymphocyte ratio to predict survival in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancers (Basel). 2019; 11:E509.

19. Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci. 2015; 16:3307–3322.

20. Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, Cheung OK, Sun H, Zeng X, Tang W, et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020; 69:365–379.

21. Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+ Ly-6G+ myeloid cells. J Immunol. 2010; 185:1383–1392.
22. Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009; 50:799–807.

23. Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015; 64:1593–1604.

24. Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, Wu K, Xia L. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015; 149:1053–1067. e14.

25. Cai H, Zhu XD, Ao JY, Ye BG, Zhang YY, Chai ZT, Wang CH, Shi WK, Cao MQ, Li XL, et al. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. OncoImmunology. 2017; 6:e1333213.

26. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253–268.

27. Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris JH, Noman MZ, Chouaib S. Critical role of tumor microenvironment in shaping NK cell functions: implication of hypoxic stress. Front Immunol. 2015; 6:482.

28. Yamamoto M, Tatsumi T, Miyagi T, Tsunematsu H, Aketa H, Hosui A, Kanto T, Hiramatsu N, Hayashi N, Takehara T. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin Exp Immunol. 2011; 165:211–219.

29. Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012; 318:154–161.

30. Muhanna N, Abu Tair L, Doron S, Amer J, Azzeh M, Mahamid M, Friedman S, Safadi R. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011; 60:90–98.

31. Zhou SL, Yin D, Hu ZQ, Luo CB, Zhou ZJ, Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019; 70:1214–1230.

32. Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014; 147:1393–1404.

33. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.

34. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, et al. LBA38_PR – CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019; 30:v874–v875.
35. Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019; 24:S3–S10.

36. Kudo M. Pembrolizumab for the treatment of hepatocellular carcinoma. Liver Cancer. 2019; 8:143–154.

37. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018; 19:940–952.
38. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020; 38:193–202.

39. Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018; 3:e000455.

40. Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Mercade TM, Tomasello G, Boisserie F, Hou J, et al. A phase 3, randomized, open-label, multicenter study to compare the efficacy and safety of tislelizumab, an anti-PD-1 antibody, versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2018; 36:TPS3110.

41. Huang J, Mo H, Wu D, Chen X, Ma L, Lan B, Qu D, Yang Q, Xu B. Phase I study of the anti-PD-1 antibody SHR-1210 in patients with advanced solid tumors. J Clin Oncol. 2017; 35:e15572.

42. Qin SK, Ren ZG, Meng ZQ, Chen ZD, Chai XL, Xiong JP, Bai YX, Yang L, Zhu H, Fang WJ, et al. LBA27 – A randomized multicentered phase II study to evaluate SHR-1210 (PD-1 antibody) in subjects with advanced hepatocellular carcinoma (HCC) who failed or intolerable to prior systemic treatment. Ann Oncol. 2018; 29:mdy424. 029.
43. Wainberg ZA, Segal NH, Jaeger D, Lee KH, Marshall J, Antonia SJ, Butler M, Sanborn RE, Nemunaitis JJ, Carlson CA, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2017; 35:4071.

44. Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. 2017; 35:4073.

45. Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, Armstrong J, Darilay A, Vlahovic G, Negro A, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin Oncol. 2018; 36:TPS4144.

46. Pishvaian MJ, Lee MS, Ryoo BY, Stein S, Lee KH, Verret W, Spahn J, Shao H, Liu B, Iizuka K, et al. LBA26 – Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018; 29:mdy424. 028.
47. Finn RS, Ducreux M, Qin S, Galle PR, Zhu AX, Ikeda M, Kim TY, Xu DZ, Verret W, Liu J, et al. IMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol. 2018; 36:TPS4141.

48. Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013; 59:81–88.

49. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017; 66:545–551.

50. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002; 415:536–541.

51. Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: a new era for cancer treatment (Review). Oncol Rep. 2019; 42:2183–2195.

52. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015; 148:1383–1391. e6.

53. Giannini EG, Aglitti A, Borzio M, Gambato M, Guarino M, Iavarone M, Lai Q, Levi Sandri GB, Melandro F, Morisco F, et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI. Cancers (Basel). 2019; 11:E1689.
54. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012; 21:309–322.

55. Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, Kaneko S, Zhu AX, Kubota T, Kraljevic S, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2018; 36:4076.

56. Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, Umeyama Y, Kamei Y, Yoshimitsu J, Fujii Y. First-line avelumab+ axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). 2019 American Society of Clinical Oncology (ASCO) Annual Meeting. May 31–Jun 4; Chicago, IL, USA. Alexandria, VA: American Society of Clinical Oncology;. 2019.
57. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019; 25:515–523.

58. Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol. 2019; 25:2416–2429.

59. Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, Chung JC, Kim HC, Lee MS, Baek MJ. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017; 49:246–254.

60. Lee HW, Cho KJ, Shin SY, Kim HY, Lee EJ, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, et al. Serum PD-1 levels change with immunotherapy response but do not predict prognosis in patients with hepatocellular carcinoma. J Liver Cancer. 2019; 19:108–116.

61. Liu CQ, Xu J, Zhou ZG, Jin LL, Yu XJ, Xiao G, Lin J, Zhuang SM, Zhang YJ, Zheng L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br J Cancer. 2018; 119:80–88.

62. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al. Nivolumab (NIVO)+ ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019; 37:4012.
63. Cheng AL, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, Kim TY, Kudo M, Breder V, Merle P, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019; 30:ix186–ix187.
Go to : 
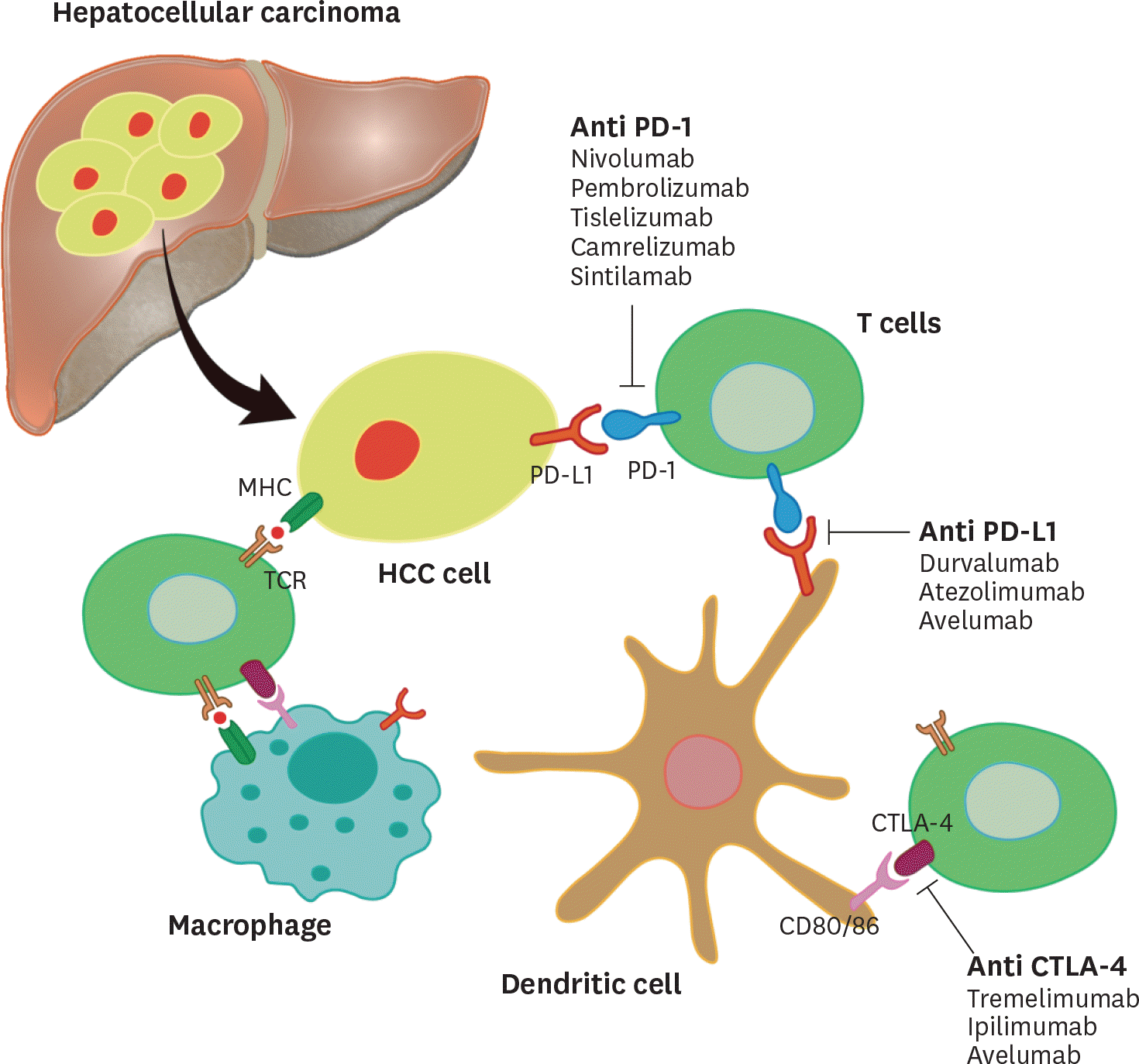 | Figure 1.Schematic diagram of T cell Interaction with hepatocellular tumor cells and dendritic cells. |
Table 1.
Clinical trials associated with ICIs in hepatocellular carcinoma
Table 2.
Summary of clinical trials of ICIs-based combination treatment in hepatocellular carcinoma
1L, first line; 2L, second line; Q3W, every 3 weeks; Q2W, every 2 weeks; Q6W, every 6 weeks; ORR, objective response rate; CR, complete response; PR, partial response; DCR, disease control rate; mOS, median overall survival; HBV, hepatitis B virus; HCV, hepatitis C virus; IV, intravenously; PFS, progression free survival; mPFS, median progression free survival; TTP, time to tumor progression; mTTP, median time to tumor progression; NA, not available; mRECIST, median Response Evaluation Criteria in Solid Tumors; BID, twice a day; SD, standard deviation; BW, body weight; QD, once daily.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download