Abstract
Aging is a complex process associated with dysregulation of the immune system and low levels of inflammation, often associated with the onset of many pathologies. The lacrimal gland (LG) plays a vital role in the maintenance of ocular physiology and changes related to aging directly affect eye diseases. The dysregulation of the immune system in aging leads to quantitative and qualitative changes in antibodies and cytokines. While there is a gradual decline of the immune system, there is an increase in autoimmunity, with a reciprocal pathway between low levels of inflammation and aging mechanisms. Elderly C57BL/6J mice spontaneously show LGs infiltration that is characterized by Th1 but not Th17 cells. The aging of the LG is related to functional alterations, reduced innervation and decreased secretory activities. Lymphocytic infiltration, destruction, and atrophy of glandular parenchyma, ductal dilatation, and secretion of inflammatory mediators modify the volume and composition of tears. Oxidative stress, the capacity to metabolize and eliminate toxic substances decreased in aging, is also associated with the reduction of LG functionality and the pathogenesis of autoimmune diseases. Although further studies are required for a better understanding of autoimmunity and aging of the LG, we described anatomic and immunology aspects that have been described so far.
References
1. Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016; 103:e29–e46.

2. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–254.

3. Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998; 102:199–209.

4. Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003; 115:278–283.

5. Nusser A, Nuber N, Wirz OF, Rolink H, Andersson J, Rolink A. The development of autoimmune features in aging mice is closely associated with alterations of the peripheral CD4+ T-cell compartment. Eur J Immunol. 2014; 44:2893–2902.
6. Ghia P, Melchers F, Rolink AG. Age-dependent changes in B lymphocyte development in man and mouse. Exp Gerontol. 2000; 35:159–165.

7. Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999; 83:737–741.

8. Zhou L, Huang LQ, Beuerman RW, Grigg ME, Li SF, Chew FT, Ang L, Stern ME, Tan D. Proteomic analysis of human tears: defensin expression after ocular surface surgery. J Proteome Res. 2004; 3:410–416.

9. Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008; 6:162–174.

10. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009; 127:763–768.
11. Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997; 124:723–728.

12. Micera A, Di Zazzo A, Esposito G, Longo R, Foulsham W, Sacco R, Sgrulletta R, Bonini S. Age-related changes to human tear composition. Invest Ophthalmol Vis Sci. 2018; 59:2024–2031.

13. Patel R, Zhu M, Robertson DM. Shifting the IGF-axis: an age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm IGF Res. 2018; 40:69–73.

14. Nien CJ, Paugh JR, Massei S, Wahlert AJ, Kao WW, Jester JV. Age-related changes in the meibomian gland. Exp Eye Res. 2009; 89:1021–1027.

15. McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009; 21:418–424.

16. Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005; 205:158–169.

17. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front Immunol. 2018; 8:1960.

19. Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009; 21:425–430.

20. Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, Larbi A, Weinberger B, Cossarizza A. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. 2016; 46:2286–2301.

21. Fulop T, Larbi A, Douziech N, Levesque I, Varin A, Herbein G. Cytokine receptor signalling and aging. Mech Ageing Dev. 2006; 127:526–537.

22. Turner JE, Brum PC. Does regular exercise counter t cell immunosenescence reducing the risk of developing cancer and promoting successful treatment of malignancies? Oxid Med Cell Longev. 2017; 2017:4234765.

23. Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005; 174:2387–2395.

24. Harpaz I, Bhattacharya U, Elyahu Y, Strominger I, Monsonego A. Old mice accumulate activated effector CD4 T cells refractory to regulatory T cell-induced immunosuppression. Front Immunol. 2017; 8:283.

25. van der Geest KS, Abdulahad WH, Tete SM, Lorencetti PG, Horst G, Bos NA, Kroesen BJ, Brouwer E, Boots AM. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014; 60:190–196.
26. Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, Whitham RH, Bakke A, Jones RE, Offner H, et al. Functional assay for human CD4+ CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003; 74:296–308.
27. Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, de Paiva CS. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2017; 10:743–756.

28. McClellan AJ, Volpe EA, Zhang X, Darlington GJ, Li DQ, Pflugfelder SC, de Paiva CS. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am J Pathol. 2014; 184:631–643.

29. Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009; 182:1247–1252.

30. Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997; 160:79–90.

31. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004; 5:133–139.

32. Kolar GR, Mehta D, Wilson PC, Capra JD. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand J Immunol. 2006; 64:314–324.

33. Banerjee M, Mehr R, Belelovsky A, Spencer J, Dunn-Walters DK. Age- and tissue-specific differences in human germinal center B cell selection revealed by analysis of IgVH gene hypermutation and lineage trees. Eur J Immunol. 2002; 32:1947–1957.

34. Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009; 8:18–25.

35. Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009; 21:201–209.

36. Nobrega A, Haury M, Gueret R, Coutinho A, Weksler ME. The age-associated increase in autoreactive immunoglobulins reflects a quantitative increase in specificities detectable at lower concentrations in young mice. Scand J Immunol. 1996; 44:437–443.

37. Mariani E, Pulsatelli L, Neri S, Dolzani P, Meneghetti A, Silvestri T, Ravaglia G, Forti P, Cattini L, Facchini A. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002; 37:219–226.
38. Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999; 47:639–646.

39. Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003; 132:24–31.

40. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017; 15:438–510.

41. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiødt M, Umehara H, Vivino F, Zhao Y, et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64:475–487.

42. Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC, Reis BL, Whitcup SM, Thompson D, Smith JA. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002; 43:2609–2614.
43. Pflugfelder SC, De Paiva CS, Moore QL, Volpe EA, Li DQ, Gumus K, Zaheer ML, Corrales RM. Aqueous tear deficiency increases conjunctival interferon-γ (IFN-γ) expression and goblet cell loss. Invest Ophthalmol Vis Sci. 2015; 56:7545–7550.

44. Pisella PJ, Brignole F, Debbasch C, Lozato PA, Creuzot-Garcher C, Bara J, Saiag P, Warnet JM, Baudouin C. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000; 107:1841–1849.

45. Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000; 41:1356–1363.
46. Baudouin C, Brignole F, Pisella PJ, De Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Adv Exp Med Biol. 2002; 506:761–769.

47. Mircheff AK, Wang Y, Jean MS, Ding C, Trousdale MD, Hamm-Alvarez SF, Schechter JE. Mucosal immunity and self-tolerance in the ocular surface system. Ocul Surf. 2005; 3:182–192.

48. Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015; 14:309–321.

49. Schein OD, Hochberg MC, Muñoz B, Tielsch JM, Bandeen-Roche K, Provost T, Anhalt GJ, West S. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999; 159:1359–1363.
50. Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, Starmer J, Wilson EM, Su MA. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. 2016; 7:11350.

51. Sullivan DA, Wickham LA, Rocha EM, Kelleher RS, da Silveira LA, Toda I. Influence of gender, sex steroid hormones, and the hypothalamic-pituitary axis on the structure and function of the lacrimal gland. Adv Exp Med Biol. 1998; 438:11–42.

53. Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013; 8:e60726.

54. Tzioufas AG, Tatouli IP, Moutsopoulos HM. Autoantibodies in Sjögren's syndrome: clinical presentation and regulatory mechanisms. Presse Med. 2012; 41:e451–e460.

55. Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Medicine (Baltimore). 2012; 91:1–9.

56. Volpe EA, Henriksson JT, Wang C, Barbosa FL, Zaheer M, Zhang X, Pflugfelder SC, de Paiva CS. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget. 2016; 7:64605–64614.

57. Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Mol Vis. 2016; 22:518–527.
58. Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology. 1995; 102:678–686.
59. Damato BE, Allan D, Murray SB, Lee WR. Senile atrophy of the human lacrimal gland: the contribution of chronic inflammatory disease. Br J Ophthalmol. 1984; 68:674–680.

60. Draper CE, Adeghate EA, Singh J, Pallot DJ. Evidence to suggest morphological and physiological alterations of lacrimal gland acini with ageing. Exp Eye Res. 1999; 68:265–276.

61. El-Fadaly AB, El-Shaarawy EA, Rizk AA, Nasralla MM, Shuaib DM. Age-related alterations in the lacrimal gland of adult albino rat: a light and electron microscopic study. Ann Anat. 2014; 196:336–351.

62. Rattan SI, Keeler KD, Buchanan JH, Holliday R. Autofluorescence as an index of ageing in human fibroblasts in culture. Biosci Rep. 1982; 2:561–567.

63. Seehafer SS, Pearce DA. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol Aging. 2006; 27:576–588.

64. Ríos JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, Zoukhri D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005; 80:477–491.

65. Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007; 84:894–904.

66. Draper CE, Adeghate E, Lawrence PA, Pallot DJ, Garner A, Singh J. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J Auton Nerv Syst. 1998; 69:173–183.

67. Marco B, Alessandro R, Philippe F, Fabio B, Paolo R, Giulio F. The effect of aging on nerve morphology and substance p expression in mouse and human corneas. Invest Ophthalmol Vis Sci. 2018; 59:5329–5335.
68. Stepp MA, Pal-Ghosh S, Tadvalkar G, Williams A, Pflugfelder SC, de Paiva CS. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp Eye Res. 2018; 169:91–98.

69. Bian F, Xiao Y, Barbosa FL, de Souza RG, Hernandez H, Yu Z, Pflugfelder SC, de Paiva CS. Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells. Mucosal Immunol. 2019. DOI: doi: 10.1038/s41385-018–0127-z.

70. Daniels PJ, Gustafson SA, French D, Wang Y, DePond W, McArthur CP. Interferon-mediated block in cell cycle and altered integrin expression in a differentiated salivary gland cell line (HSG) cultured on Matrigel. J Interferon Cytokine Res. 2000; 20:1101–1109.

71. Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, Tzioufas AG, Baer AN, Rosen A. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012; 109:17609–17614.

72. García-Posadas L, Hodges RR, Li D, Shatos MA, Storr-Paulsen T, Diebold Y, Dartt DA. Interaction of IFN-γ with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016; 9:206–217.

73. De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007; 48:2553–2560.
74. Pitcher JD 3rd, De Paiva CS, Pelegrino FS, McClellan AJ, Raince JK, Pangelinan SB, Rahimy E, Farley WJ, Stern ME, Li DQ, et al. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2011; 52:3221–3227.

75. Xiao B, Wang Y, Reinach PS, Ren Y, Li J, Hua S, Lu H, Chen W. Dynamic ocular surface and lacrimal gland changes induced in experimental murine dry eye. PLoS One. 2015; 10:e0115333.

76. Bacman S, Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 1998; 39:151–156.
77. Bluestone R, Easty DL, Goldberg LS, Jones BR, Pettit TH. Lacrimal immunoglobulins and complement quantified by counter-immunoelectrophoresis. Br J Ophthalmol. 1975; 59:279–281.

78. Sullivan DA, Hann LE. Hormonal influence on the secretory immune system of the eye: endocrine impact on the lacrimal gland accumulation and secretion of IgA and IgG. J Steroid Biochem. 1989; 34:253–262.

79. You IC, Bian F, Volpe EA, de Paiva CS, Pflugfelder SC. Age-related conjunctival disease in the C57BL/6. NOD-Aec1Aec2 mouse model of Sjögren syndrome develops independent of lacrimal dysfunction. Invest Ophthalmol Vis Sci. 2015; 56:2224–2233.
80. Sullivan DA, Hann LE, Yee L, Allansmith MR. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol (Copenh). 1990; 68:188–194.

81. Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013; 183:35–48.

82. Marcozzi G, Liberati V, Madia F, Centofanti M, de Feo G. Age- and gender-related differences in human lacrimal fluid peroxidase activity. Ophthalmologica. 2003; 217:294–297.

83. Nava A, Barton K, Monroy DC, Pflugfelder SC. The effects of age, gender, and fluid dynamics on the concentration of tear film epidermal growth factor. Cornea. 1997; 16:430–438.

84. Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009; 147:198–205.

85. Batista TM, Tomiyoshi LM, Dias AC, Roma LP, Módulo CM, Malki LT, Filho EB, Deminice R, Jordão AA Jr, Cunha DA, et al. Age-dependent changes in rat lacrimal gland anti-oxidant and vesicular related protein expression profiles. Mol Vis. 2012; 18:194–202.
86. Benlloch-Navarro S, Franco I, Sánchez-Vallejo V, Silvestre D, Romero FJ, Miranda M. Lipid peroxidation is increased in tears from the elderly. Exp Eye Res. 2013; 115:199–205.

87. Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011; 90:830–840.

88. Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017; 23:174–184.

89. Mangano EN, Litteljohn D, So R, Nelson E, Peters S, Bethune C, Bobyn J, Hayley S. Interferon-γ plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. 2012; 33:1411–1426.

90. Pinazo-Durán MD, Gallego-Pinazo R, García-Medina JJ, Zanón-Moreno V, Nucci C, Dolz-Marco R, Martínez-Castillo S, Galbis-Estrada C, Marco-Ramírez C, López-Gálvez MI, et al. Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging. 2014; 9:637–652.
91. Choi W, Lian C, Ying L, Kim GE, You IC, Park SH, Yoon KC. Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-sjogren syndrome: potential biomarkers for dry eye disease. Curr Eye Res. 2016; 41:1143–1149.

92. Deng R, Hua X, Li J, Chi W, Zhang Z, Lu F, Zhang L, Pflugfelder SC, Li DQ. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One. 2015; 10:e0126561.

93. Uchino Y, Kawakita T, Ishii T, Ishii N, Tsubota K. A new mouse model of dry eye disease: oxidative stress affects functional decline in the lacrimal gland. Cornea. 2012; 31(Suppl 1):S63–S67.
Figure 1.
Aging is accompanied by a systemic increase in Igs. Sera from 8-week-old (8W) and 15-month-old (15M) female C57BL/6J mice were collected by cardiac puncture upon euthanasia and Igs were measured using Luminex assay (Mann-Whitney U test).
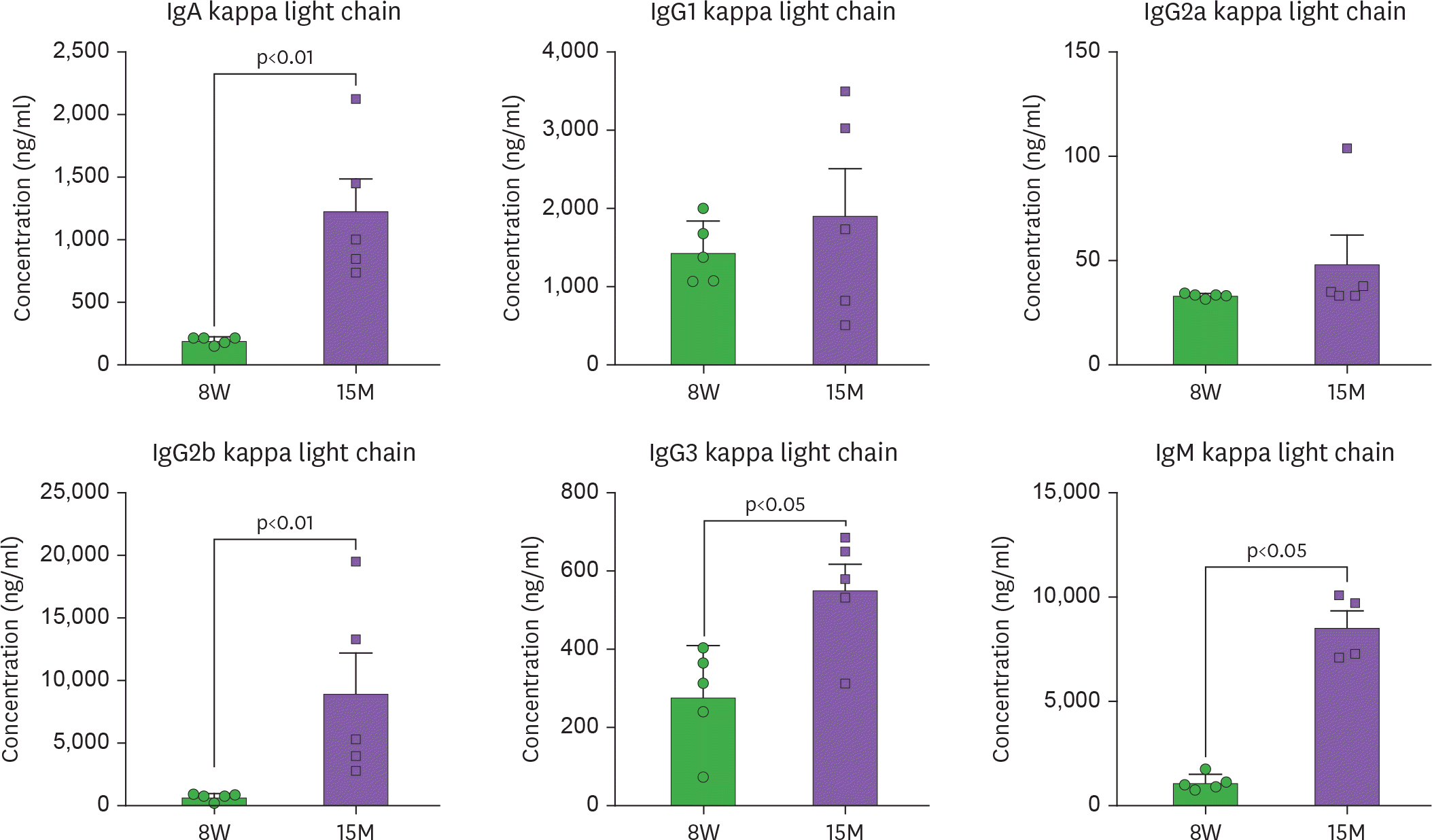
Figure 2.
Pathological changes to the aged lacrimal gland (LG). (A) Macro images of 8-week (8W) and 24 months old (24M) female LG of C57BL/6 mice. Arrow heads indicate cysts. (B) Representative images of lacrimal gland sections stained with H&E. Areas of lymphocytic infiltration are demarcated in the 24M section. (C) Right and left LG wet weight/body ratio (n=19/group). One-way ANOVA followed by Sidak's multiple comparison test. * Asterisks indicate enlarged ducts.
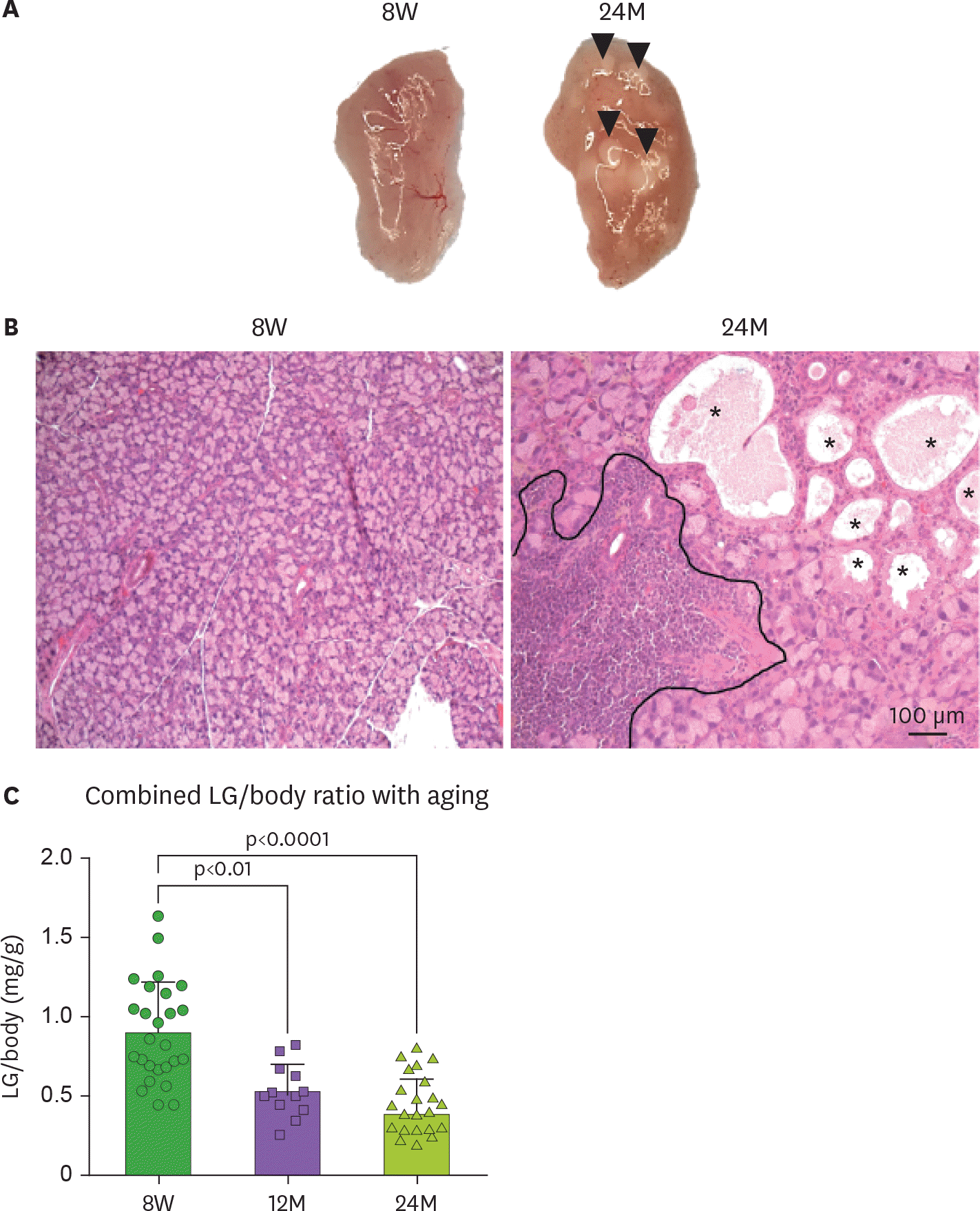
Figure 3.
Transmission electron microscopic examination of lacrimal gland acinar of young (8W) and aged (24M) C57BL/6J female mice. Frequent marked structural changes in mitochondria (see insets) in aged mice were observed, including swelling and loss of cristae and disorganization. Increased number of mucous-containing granules were also observed (bar=04 µm). 8W, 8 weeks of age; 24M, 24 months of age.
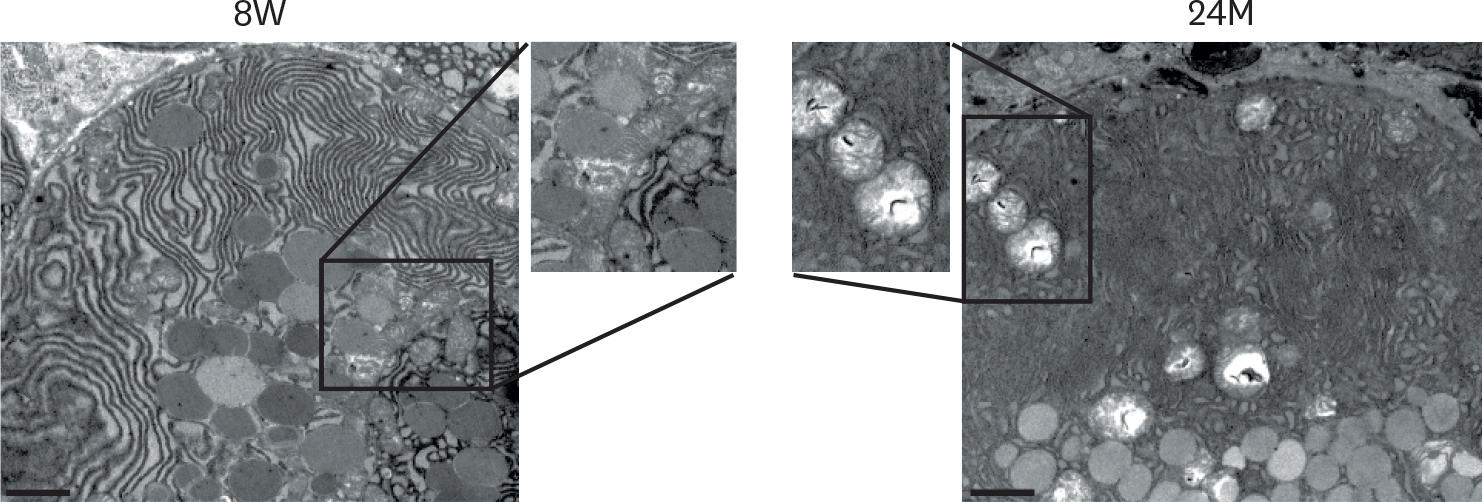
Figure 4.
Lipofuscin and lipofuscin-like structures are increased in female aged C57BL/6J lacrimal gland. Paraffin-embedded lacrimal gland histologic sections were fixed in formalin and routinely processed in paraffin and stained with H&E. The same area was photographed with a color camera or with a fluorescent camera with the indicated filters. Autofluorescence in ducts is visible with the 488 and 594 filters, while distinct structures are autofluorescent with the 594 and CY5 filters. (bar=25 µm). 8W, 8 weeks of age; 24M, 24 months of age.

Table 1.
Tear parameters in young and aged female C57B/6J mice
| Parameter/age | 8W | 24M | p value |
|---|---|---|---|
| Tear volume* (µL) (n=8) | 0.07±0.01 | 0.15±0.07 | 0.0005 |
| Body weight (g) (n=8) | 20.0±1.12 | 40.3±6.70 | 0.01 |
| Tear volume/body weight* (µL/g) (n=8) | 0.0034±0.0007 | 0.0037±0.0010 | 0.65 |
| Tear IgA† (pg/ml, n=4–8) | 1,237±1,279 | 9,731±4,099 | 0.0006 |
| Tear IgM† (pg/mL, n=4–8) | 3.3±3.0 | 2,580±2,300 | 0.007 |
| IgA/IgM ratio† (n=4–8) | 325.8±250.8 | 4.53±2.7 | 0.03 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download