Abstract
Bone marrow-derived dendritic cells (BM-DCs) are generated from bone marrow (BM) cells cultured with granulocyte macrophage-colony stimulating factor (GM-CSF) for a week. In this study we investigated the effect of duration on the BM culture with GM-CSF. Within several months, the cells in the BM culture gradually expressed homogeneous levels of CD11c and major histocompatibility complex II on surface, and they became unable to stimulate allogeneic naïve T cells in mixed lymphocyte reaction (MLR). In addition, when the BM culture were sustained for 32 wk or longer, the BM cells acquired ability to suppress the proliferation of allogeneic T cells in MLR as well as the response of ovalbumin-specific OT-I transgenic T cells in antigen-dependent manner. We found that, except for programmed death-ligand 1, most cell surface molecules were expressed lower in the BM cells cultured with GM-CSF for the extended duration. These results indicate that BM cells in the extended culture with GM-CSF undergo 2 distinct steps of functional change; first, they lose the immunostimulatory capacity; and next, they gain the immunosuppressive ability.
References
1. Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978; 75:5132–5136.

2. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012; 30:1–22.

3. Park CG. Vaccine strategies utilizing C-type lectin receptors on dendritic cells in vivo. Clin Exp Vaccine Res. 2014; 3:149–154.
4. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006; 311:83–87.

5. Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009; 324:392–397.
6. Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209 (+) dendritic cells for immune T cell areas. Cell. 2010; 143:416–429.

7. Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Curr Protoc Immunol. 2009. ;Chapter 3: Unit 3.7.

8. Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999; 223:77–92.

9. Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997; 158:2723–2730.
10. Ebihara S, Endo S, Ito K, Ito Y, Akiyama K, Obinata M, Takai T. Immortalized dendritic cell line with efficient cross-priming ability established from transgenic mice harboring the temperature-sensitive SV40 large T-antigen gene. J Biochem. 2004; 136:321–328.

11. Peng S, Kim TW, Lee JH, Yang M, He L, Hung CF, Wu TC. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005; 16:584–593.

12. Kang TH, Lee JH, Bae HC, Noh KH, Kim JH, Song CK, Shin BC, Hung CF, Wu TC, Park JS, et al. Enhancement of dendritic cell-based vaccine potency by targeting antigen to endosomal/lysosomal compartments. Immunol Lett. 2006; 106:126–134.

13. Ichiyanagi T, Imai T, Kajiwara C, Mizukami S, Nakai A, Nakayama T, Udono H. Essential role of endogenous heat shock protein 90 of dendritic cells in antigen cross-presentation. J Immunol. 2010; 185:2693–2700.

14. Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009; 46:1229–1239.

15. Dolan BP, Li L, Takeda K, Bennink JR, Yewdell JW. Defective ribosomal products are the major source of antigenic peptides endogenously generated from influenza A virus neuraminidase. J Immunol. 2010; 184:1419–1424.

16. He T, Tang C, Xu S, Moyana T, Xiang J. Interferon gamma stimulates cellular maturation of dendritic cell line DC2.4 leading to induction of efficient cytotoxic T cell responses and antitumor immunity. Cell Mol Immunol. 2007; 4:105–111.
17. Ryu SH, Na HY, Sohn M, Han SM, Choi W, In H, Hong S, Jeon H, Seo JY, Ahn J, et al. Reduced expression of granule proteins during extended survival of eosinophils in splenocyte culture with GM-CSF. Immunol Lett. 2016; 173:7–20.

18. Han SM, Na HY, Ham O, Choi W, Sohn M, Ryu SH, In H, Hwang KC, Park CG. TCF4-targeting miR-124 is differentially expressed amongst dendritic cell subsets. Immune Netw. 2016; 16:61–74.

19. Ryu SH, Na HY, Sohn M, Choi W, In H, Shin HS, Choi JH, Park CG. Competent antigen-presenting cells are generated from the long-term culture of splenocytes with granulocyte-macrophage colony-stimulating factor. Immunol Lett. 2017; 188:96–107.

20. Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015; 42:1197–1211.

Figure 1.
Cells from the extended cultures of BM with GM-CSF lose their capacity to stimulate allogeneic T cells. BM cells isolated from C57BL/6 mouse are cultured with GM-CSF, and harvested in 4-wk intervals. The 5×104 CFSE-labeled allogeneic T cells from BALB/c mouse are co-cultured with 1.7×104 BM cells derived respectively from (A) 4-, and (B) 8-wk old cultures in comparison with the 1-wk old culture. After 4 days, CFSE low T cells are analyzed by a flow cytometer and counted. Representative data are shown from at least 2 independent experiments in quadruplicate. Error bars indicate mean ± standard error of the mean across quadruplicate samples. ** p≤0.01, *** p≤0.001.
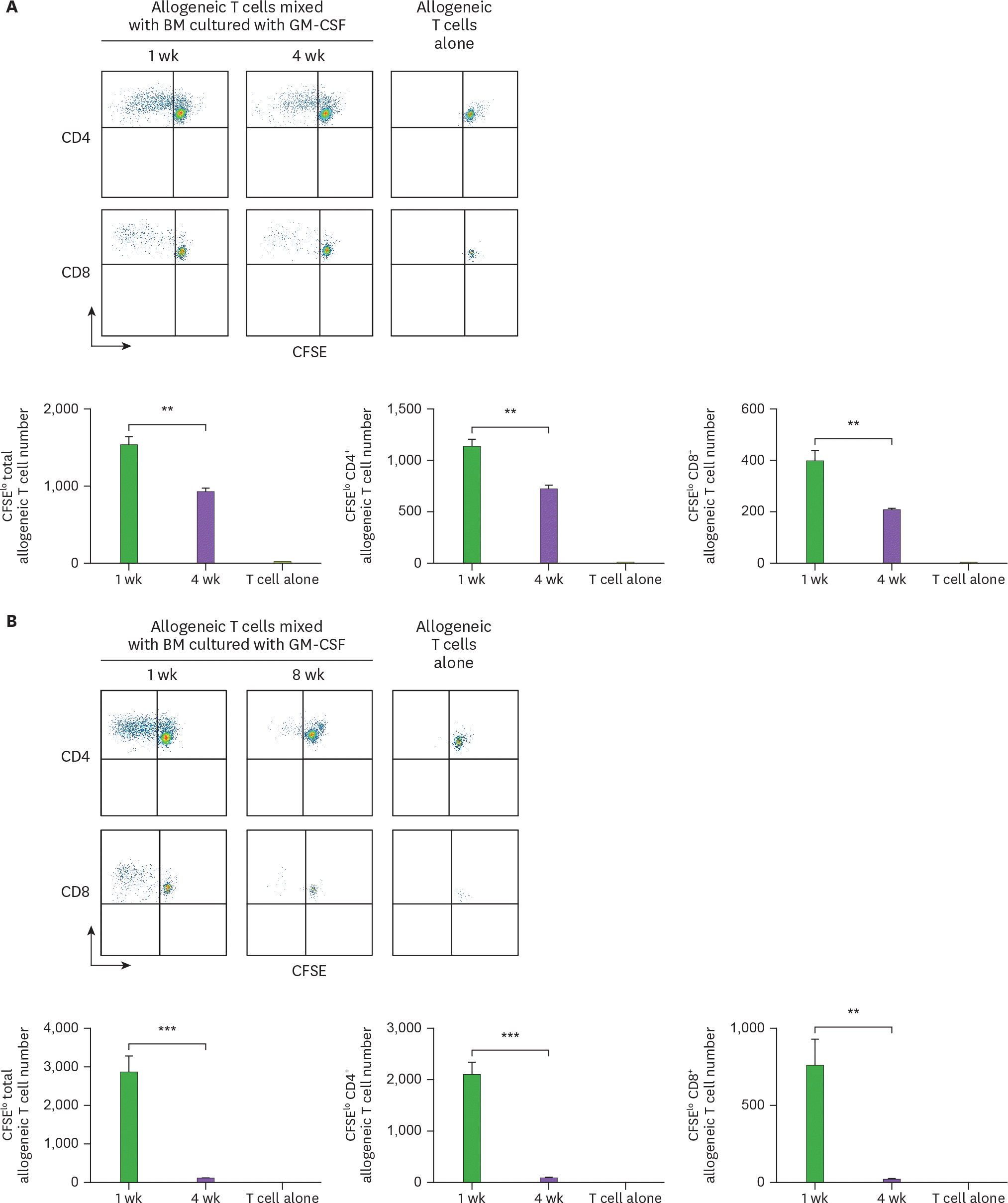
Figure 2.
Long-term cultured BM cells retain homogeneous levels of CD11c and MHC II. BM cells cultured with GM-CSF for 1 wk versus (A) 4 wk or (B) 57 wk are compared for their MHC II and CD11c expression. Representative data are shown from at least 2 independent experiments.
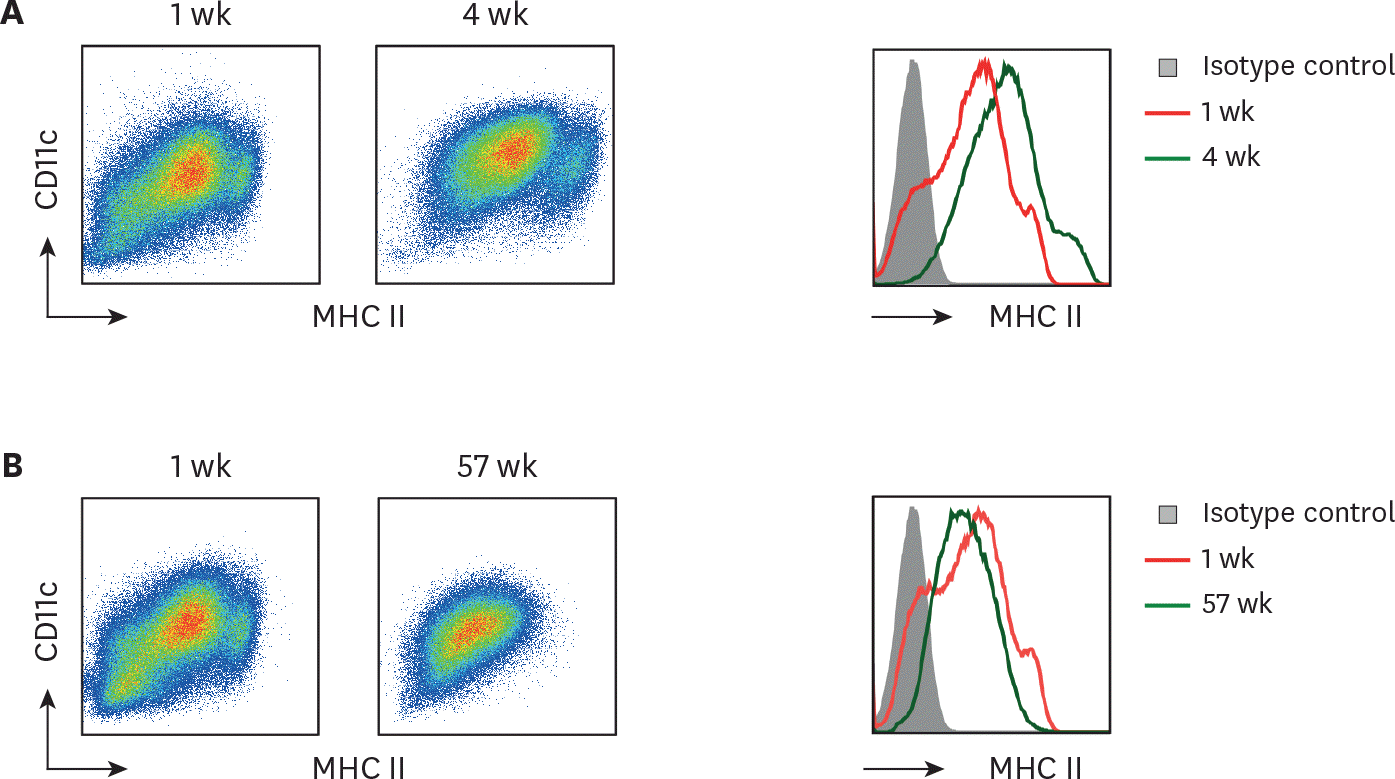
Figure 3.
The expression of surface molecules is evaluated on BM cells in the extended cultures with GM-CSF. BM cells in the 1-wk and 70-wk old cultures are stained side by side with antibodies against the indicated molecules. (A) In the 1-wk old BM culture, CD11c+ MHC II hi cells are gated as DCs in red tetragon and CD11c+ MHC II int cells are gated as macrophages in purple tetragon. The whole BM cells in the 70-wk old culture are gated for analysis. (B) Histograms illustrate the staining of a respective antibody (open red line) versus an isotype control (area filled with gray). The expression of each molecule is detected on cell surface but IDO-1 detected intracellularly. Mean fluorescent index is denoted in each histogram. Representative data are shown from at least 2 independent experiments.
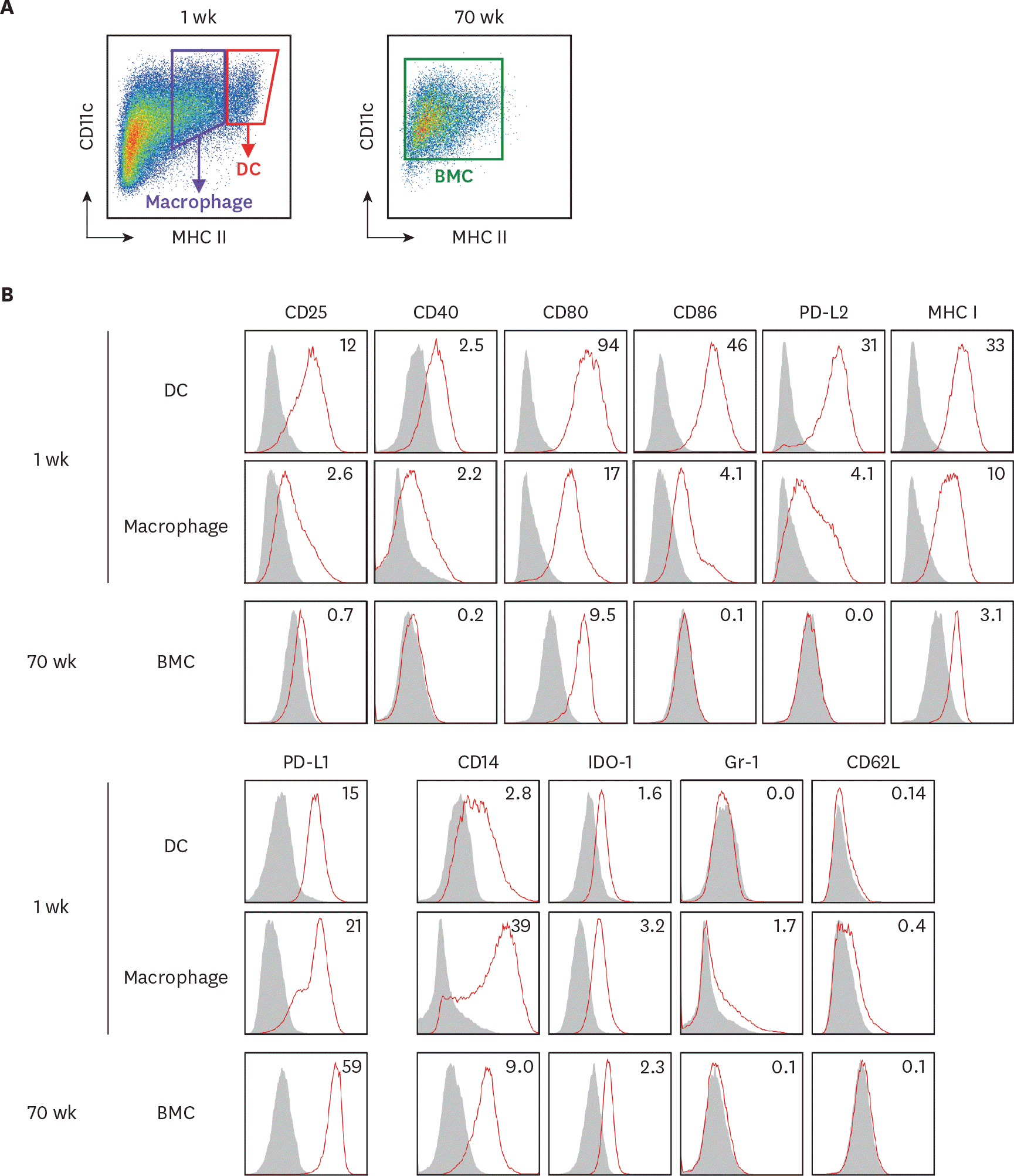
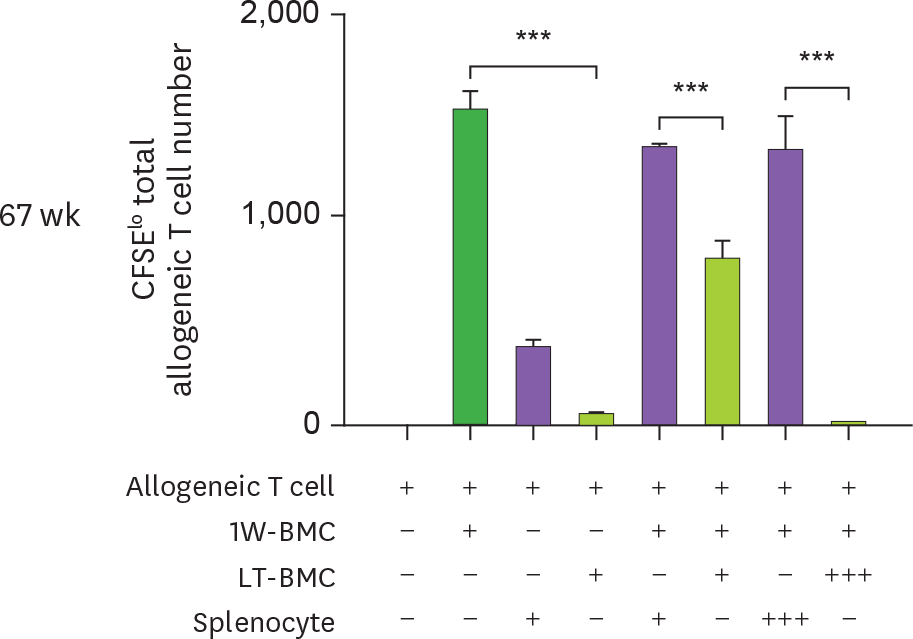
Figure 4.
Cells from the extended cultures of BM with GM-CSF are suppressive to MLR. (A) BM cells cultured for 1 wk (1W-BMC) were mixed with either control syngeneic splenocytes or BM cells cultured long-term (LT-BMC) for 16 or 32 wk and co-cultured with 5×104 CFSE-labeled allogeneic T cells isolated from BALB/c mice. After 4 days, CFSE low allogeneic T cells are assessed. Representative flow cytograms are shown from 2 independent experiments in quadruplicate. (B) The suppressor cell activity of BM cells in extended cultures at different time points is assessed as in (A). Assays are performed at the ratios of 1(+):1(+):3(+) and 1(+):3(+++):3(+) for 1W-BMC:LT-BMC/Splenocyte:Allogeneic T cell. The number of CFSE low T cells were counted and plotted in graphs. Data are generated from 2 independent experiments in quadruplicate. Error bars indicate mean ± standard error of the mean across quadruplicate samples. * p≤0.05, ** p≤0.01, *** p≤0.001.
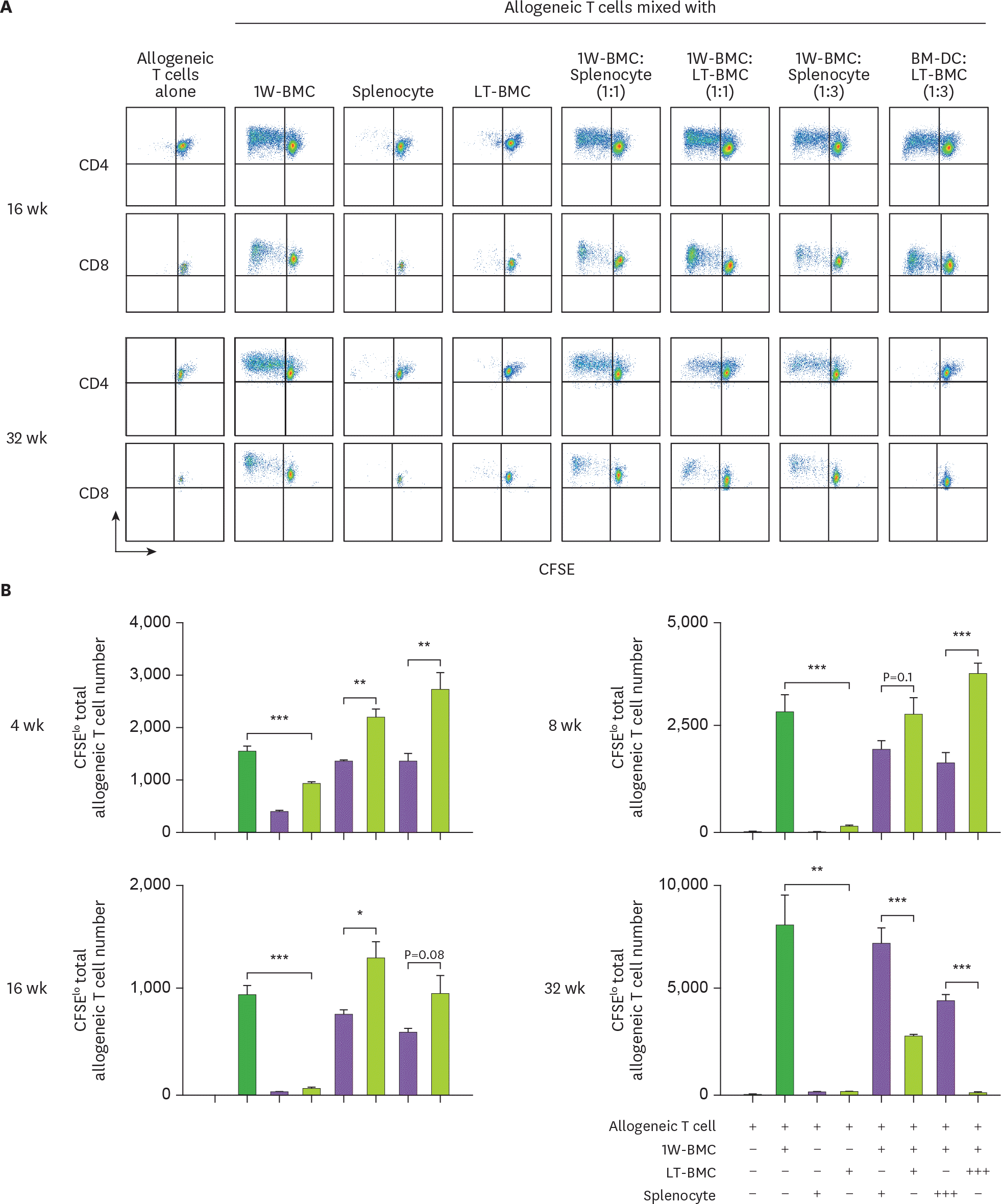
Figure 5.
Cells from the extended culture of BM with GM-CSF are suppressive to the proliferation of OT-I T cells in antigen-dependent manner. (A) BM cells cultured for 1 wk (1W-BMC), 60-wk old BM cells cultured long-term (LT-BMC), and control syngeneic splenocytes are pre-treated with OVA (100 μg/ml), mixed as indicated, co-cultured with 105 CFSE-labeled OT-I T cells for 3 days, and analyzed for the number CFSE low OT-I T cells. (B) The suppressor cell activity is assessed as in (A) except that 62-wk old LT-BMC and control syngeneic splenocytes are not pre-treated with OVA. Assays are performed at the ratios of 1(+):1(+):100(+), 1(+):3(++):100(+), and 1(+):10(+++):100(+) for 1W-BMC:LT-BMC/Splenocyte:OT-I T cell. The number of CFSE low T cells were counted and plotted in graphs. Data are generated from 2 independent experiments in triplicate. Error bars indicate mean ± standard error of the mean across triplicate samples. NS, not significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
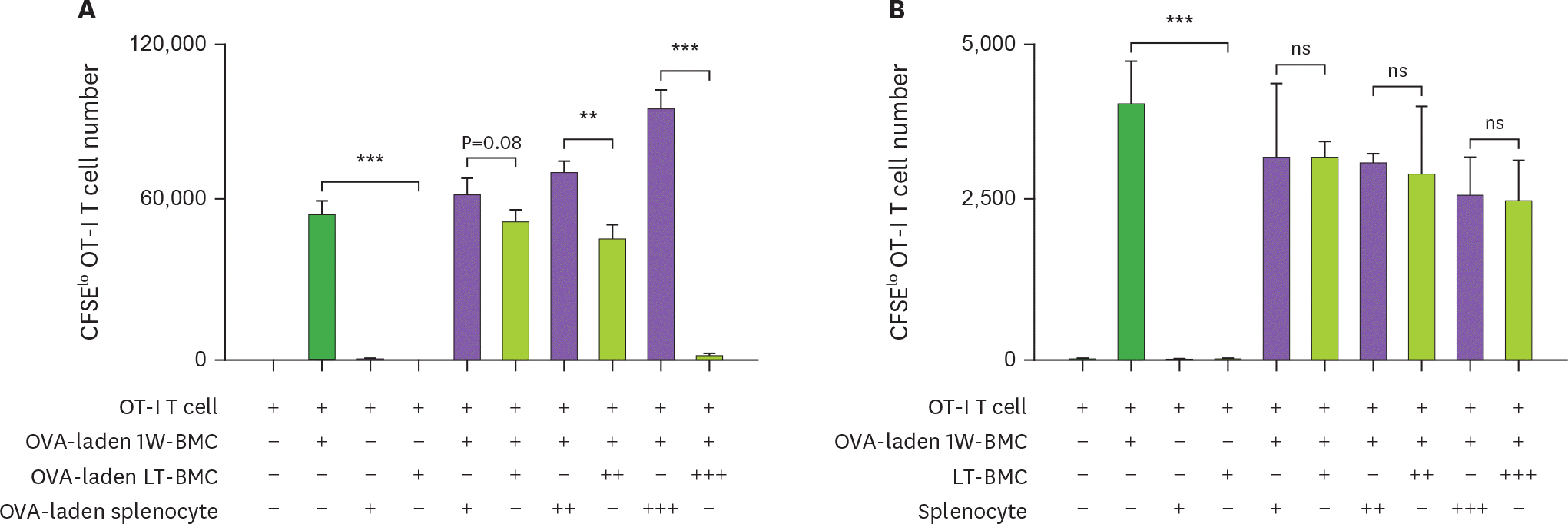
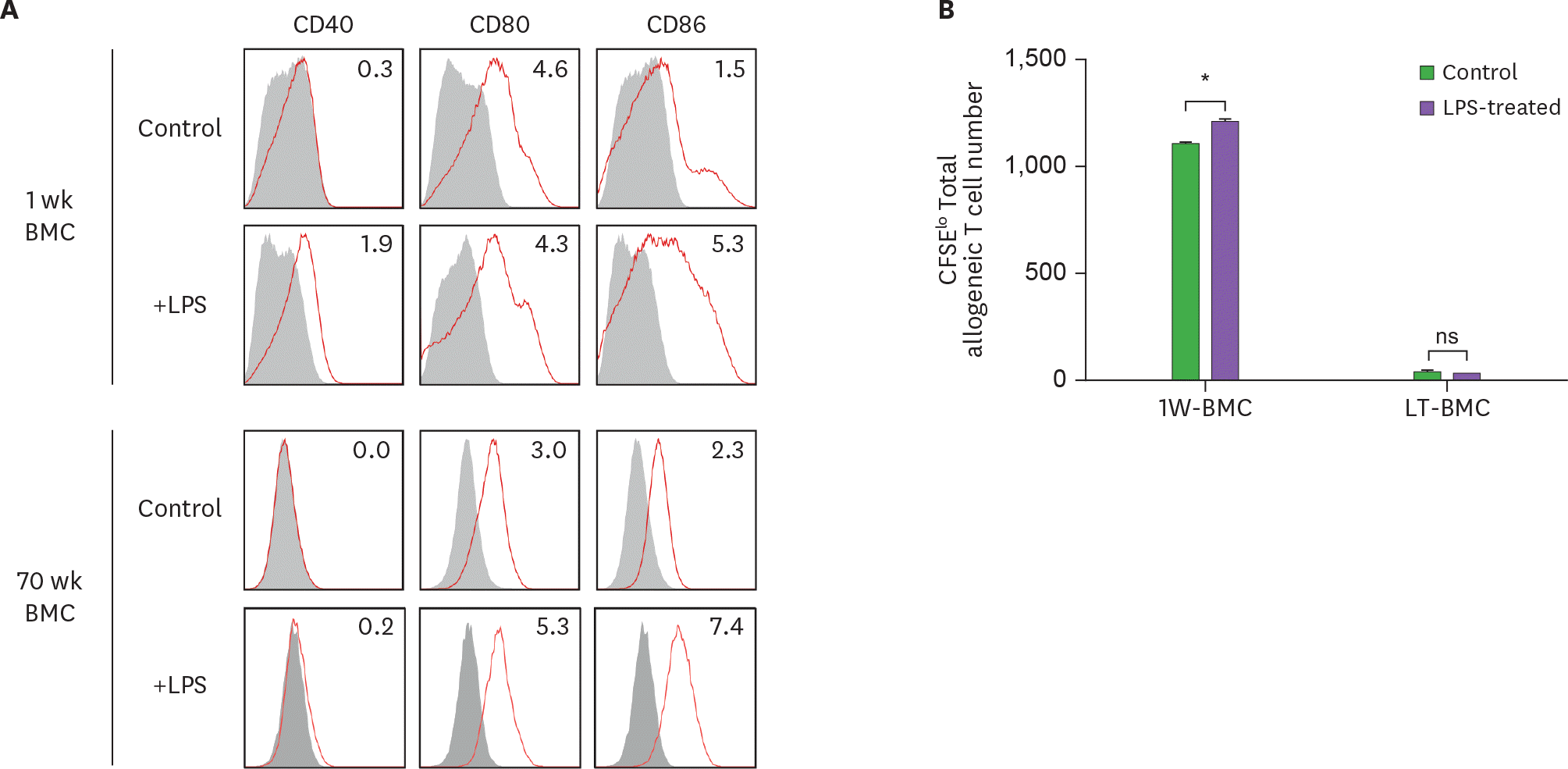
Figure 6.
LPS stimulation does not affect the stimulatory function of BM cells cultured long-term (LT-BMC). BM cells in the 1-wk and 70-wk old cultures are stimulated side by side with LPS (100 μg/ml) for 18 h. After LPS stimulation, BM cells in the respective culture are stained with antibodies against the indicated molecules (A) or co-cultured with 5×104 CFSE-labeled allogeneic T cells for MLR (B). Data are generated from 2 independent experiments in quadruplicate. Error bars indicate mean ± standard error of the mean across quadruplicate samples. 1W-BMC, BM cells cultured for 1 wk; NS, not significant. * p≤0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download