Abstract
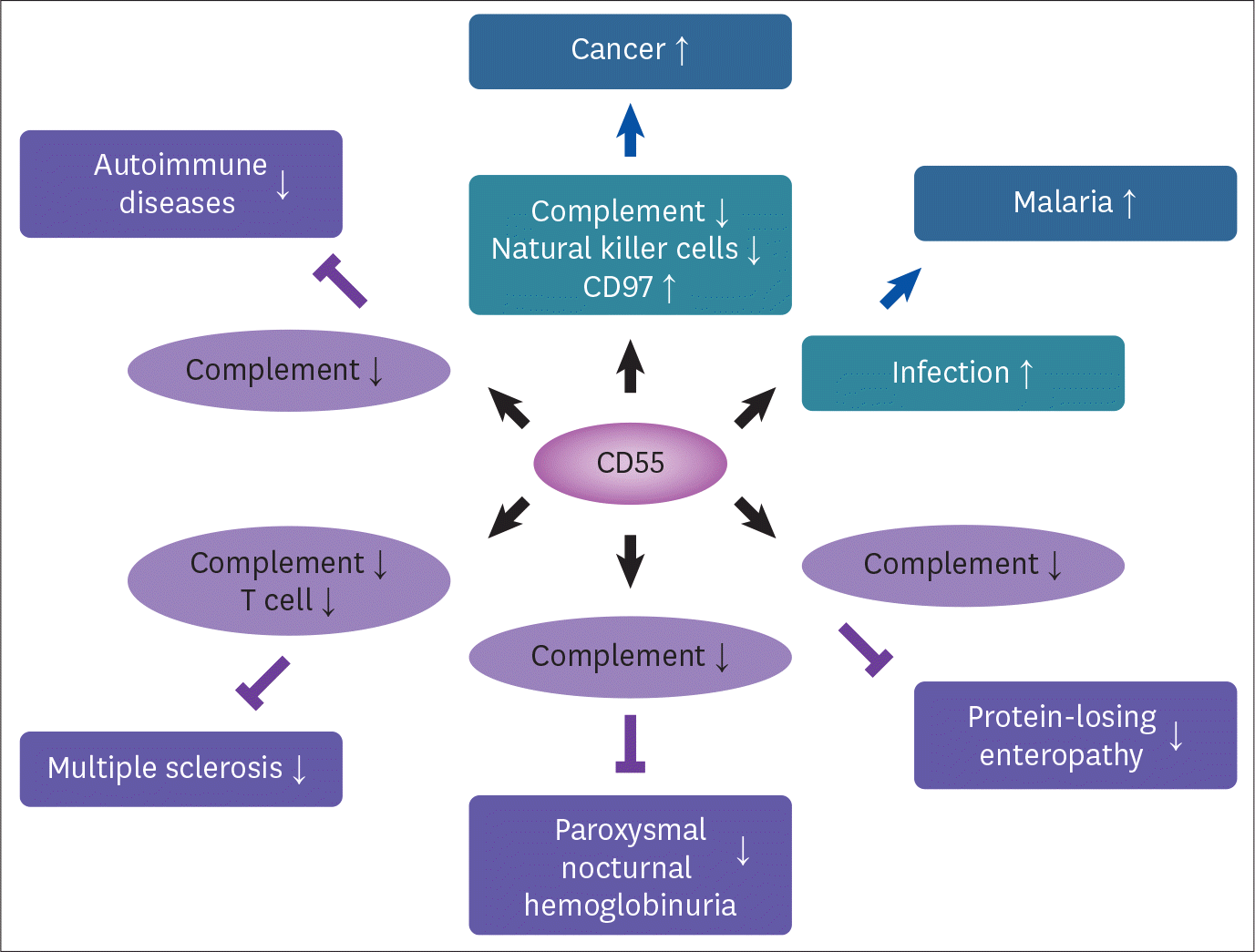
The complement is a part of the immune system that plays several roles in removing pathogens. Despite the importance of the complement system, the exact role of each component has been overlooked because the complement system was thought to be a nonspecific humoral immune mechanism that worked against pathogens. Decay-accelerating factor (DAF or CD55) is a known inhibitor of the complement system and has recently attracted substantial attention due to its role in various diseases, such as cancer, protein-losing enteropathy, and malaria. Some protein-losing enteropathy cases are caused by CD55 deficiency, which leads to complement hyperactivation, malabsorption, and angiopathic thrombosis. In addition, CD55 has been reported to be an essential host receptor for infection by the malaria parasite. Moreover, CD55 is a ligand of the seven-span transmembrane receptor CD97. Since CD55 is present in various cells, the functional role of CD55 has been expanded by showing that CD55 is associated with a variety of diseases, including cancer, malaria, protein-losing enteropathy, paroxysmal nocturnal hemoglobinuria, and autoimmune diseases. This review summarizes the current understanding of CD55 and the role of CD55 in these diseases. It also provides insight into the development of novel drugs for the diagnosis and treatment of diseases associated with CD55.
Go to : 
References
1. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010; 18:843–851.

2. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013; 342:1432–1433.
3. Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007; 111:6–13.

4. Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol. 2013; 25:54–64.

5. Lukacik P, Roversi P, White J, Esser D, Smith GP, Billington J, Williams PA, Rudd PM, Wormald MR, Harvey DJ, et al. Complement regulation at the molecular level: the structure of decay-accelerating factor. Proc Natl Acad Sci U S A. 2004; 101:1279–1284.
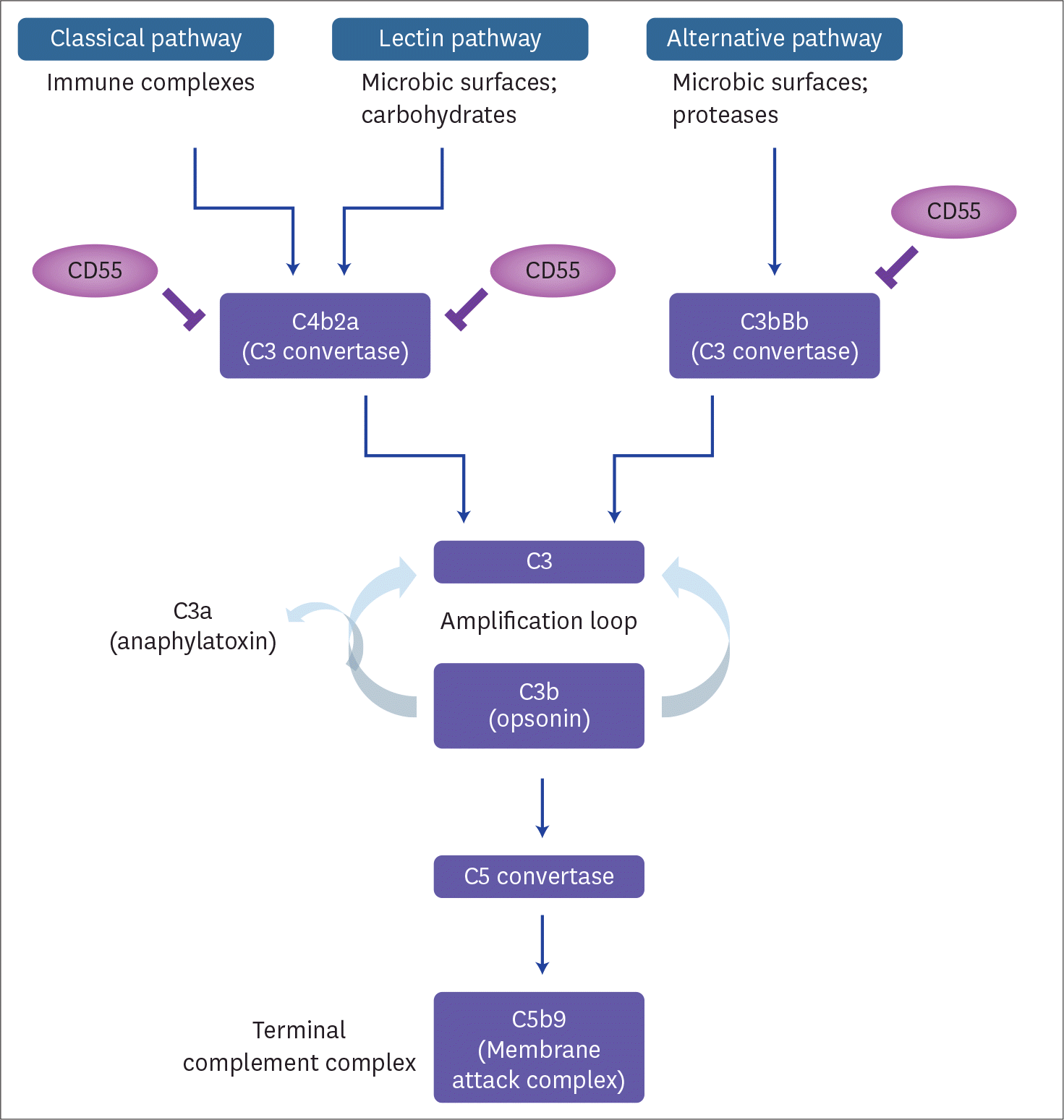
6. Trouw LA, Daha MR. Role of complement in innate immunity and host defense. Immunol Lett. 2011; 138:35–37.

7. Kolev M, Towner L, Donev R. Complement in cancer and cancer immunotherapy. Arch Immunol Ther Exp (Warsz). 2011; 59:407–419.

8. Mikesch JH, Schier K, Roetger A, Simon R, Buerger H, Brandt B. The expression and action of decay-accelerating factor (CD55) in human malignancies and cancer therapy. Cell Oncol. 2006; 28:223–232.

9. Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989; 7:35–58.

10. Ozen A, Comrie WA, Ardy RC, Dominguez Conde C, Dalgic B, Beser OF, Morawski AR, Karakoc-Aydiner E, Tutar E, Baris S, et al. CD55 deficiency, early-onset protein-losing enteropathy, and thrombosis. N Engl J Med. 2017; 377:52–61.

11. Kurolap A, Eshach-Adiv O, Hershkovitz T, Paperna T, Mory A, Oz-Levi D, Zohar Y, Mandel H, Chezar J, Azoulay D, et al. Loss of CD55 in eculizumab-responsive protein-losing enteropathy. N Engl J Med. 2017; 377:87–89.

12. Egan ES, Jiang RH, Moechtar MA, Barteneva NS, Weekes MP, Nobre LV, Gygi SP, Paulo JA, Frantzreb C, Tani Y, et al. Malaria. A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion. Science. 2015; 348:711–714.
13. Hoffmann EM. Inhibition of complement by a substance isolated from human erythrocytes. II. Studies on the site and mechanism of action. Immunochemistry. 1969; 6:405–419.
15. Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987; 165:848–864.

16. Miwa T, Sun X, Ohta R, Okada N, Harris CL, Morgan BP, Song WC. Characterization of glycosylphosphatidylinositol-anchored decay accelerating factor (GPI-DAF) and transmembrane DAF gene expression in wild-type and GPI-DAF gene knockout mice using polyclonal and monoclonal antibodies with dual or single specificity. Immunology. 2001; 104:207–214.

17. Kwon YC, Kim H, Meyer K, Di Bisceglie AM, Ray R. Distinct CD55 isoform synthesis and inhibition of complement-dependent cytolysis by hepatitis C virus. J Immunol. 2016; 197:1127–1136.

18. Morgan J, Spendlove I, Durrant LG. The role of CD55 in protecting the tumour environment from complement attack. Tissue Antigens. 2002; 60:213–223.

19. Vainer ED, Meir K, Furman M, Semenenko I, Konikoff F, Vainer GW. Characterization of novel CD55 isoforms expression in normal and neoplastic tissues. Tissue Antigens. 2013; 82:26–34.
20. Caras IW, Davitz MA, Rhee L, Weddell G, Martin DW Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987; 325:545–549.

21. Nakagawa M, Mizuno M, Kawada M, Uesu T, Nasu J, Takeuchi K, Okada H, Endo Y, Fujita T, Tsuji T. Polymorphic expression of decay-accelerating factor in human colorectal cancer. J Gastroenterol Hepatol. 2001; 16:184–189.

22. Kuraya M, Yefenof E, Klein G, Klein E. Expression of the complement regulatory proteins CD21, CD55 and CD59 on Burkitt lymphoma lines: their role in sensitivity to human serum-mediated lysis. Eur J Immunol. 1992; 22:1871–1876.

23. Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001; 19:451–455.

24. Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005; 201:567–577.
25. Capasso M, Durrant LG, Stacey M, Gordon S, Ramage J, Spendlove I. Costimulation via CD55 on human CD4+ T cells mediated by CD97. J Immunol. 2006; 177:1070–1077.
26. Finberg RW, White W, Nicholson-Weller A. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J Immunol. 1992; 149:2055–2060.
27. Abbott RJ, Spendlove I, Roversi P, Fitzgibbon H, Knott V, Teriete P, McDonnell JM, Handford PA, Lea SM. Structural and functional characterization of a novel T cell receptor co-regulatory protein complex, CD97-CD55. J Biol Chem. 2007; 282:22023–22032.

28. Visser L, de Vos AF, Hamann J, Melief MJ, van Meurs M, van Lier RA, Laman JD, Hintzen RQ. Expression of the EGF-TM7 receptor CD97 and its ligand CD55 (DAF) in multiple sclerosis. J Neuroimmunol. 2002; 132:156–163.

29. Aust G, Eichler W, Laue S, Lehmann I, Heldin NE, Lotz O, Scherbaum WA, Dralle H, Hoang-Vu C. CD97: a dedifferentiation marker in human thyroid carcinomas. Cancer Res. 1997; 57:1798–1806.
30. Mustafa T, Klonisch T, Hombach-Klonisch S, Kehlen A, Schmutzler C, Koehrle J, Gimm O, Dralle H, Hoang-Vu C. Expression of CD97 and CD55 in human medullary thyroid carcinomas. Int J Oncol. 2004; 24:285–294.

31. Steinert M, Wobus M, Boltze C, Schutz A, Wahlbuhl M, Hamann J, Aust G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am J Pathol. 2002; 161:1657–1667.

32. Safaee M, Clark AJ, Oh MC, Ivan ME, Bloch O, Kaur G, Sun MZ, Kim JM, Oh T, Berger MS, et al. Overexpression of CD97 confers an invasive phenotype in glioblastoma cells and is associated with decreased survival of glioblastoma patients. PLoS One. 2013; 8:e62765.

33. Wu J, Lei L, Wang S, Gu D, Zhang J. Immunohistochemical expression and prognostic value of CD97 and its ligand CD55 in primary gallbladder carcinoma. J Biomed Biotechnol. 2012; 2012:587672.

34. He Y, Wang W, Xu L, Li L, Liu J, Feng M, Bu H. Immunohistochemical expression and prognostic significance of CD97 and its ligand DAF in human cervical squamous cell carcinoma. Int J Gynecol Pathol. 2015; 34:473–479.

35. Aust G, Steinert M, Schutz A, Boltze C, Wahlbuhl M, Hamann J, Wobus M. CD97, but not its closely related EGF-TM7 family member EMR2, is expressed on gastric, pancreatic, and esophageal carcinomas. Am J Clin Pathol. 2002; 118:699–707.

36. Mustafa T, Eckert A, Klonisch T, Kehlen A, Maurer P, Klintschar M, Erhuma M, Zschoyan R, Gimm O, Dralle H, et al. Expression of the epidermal growth factor seven-transmembrane member CD97 correlates with grading and staging in human oral squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev. 2005; 14:108–119.
37. Safaee M, Clark AJ, Ivan ME, Oh MC, Bloch O, Sun MZ, Oh T, Parsa AT. CD97 is a multifunctional leukocyte receptor with distinct roles in human cancers (Review). Int J Oncol. 2013; 43:1343–1350.

38. Wang T, Ward Y, Tian L, Lake R, Guedez L, Stetler-Stevenson WG, Kelly K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood. 2005; 105:2836–2844.

39. Han SL, Xu C, Wu XL, Li JL, Liu Z, Zeng QQ. The impact of expressions of CD97 and its ligand CD55 at the invasion front on prognosis of rectal adenocarcinoma. Int J Colorectal Dis. 2010; 25:695–702.

40. Hamann J, Hartmann E, van Lier RA. Structure of the human CD97 gene: exon shuffling has generated a new type of seven-span transmembrane molecule related to the secretin receptor superfamily. Genomics. 1996; 32:144–147.

41. He Z, Wu H, Jiao Y, Zheng J. Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol Lett. 2015; 9:793–797.

42. Lin HH, Stacey M, Saxby C, Knott V, Chaudhry Y, Evans D, Gordon S, McKnight AJ, Handford P, Lea S. Molecular analysis of the epidermal growth factor-like short consensus repeat domain-mediated protein-protein interactions: dissection of the CD97-CD55 complex. J Biol Chem. 2001; 276:24160–24169.
43. Meng ZW, Liu MC, Hong HJ, Du Q, Chen YL. Expression and prognostic value of soluble CD97 and its ligand CD55 in intrahepatic cholangiocarcinoma. Tumour Biol. 2017; 39:1010428317694319.

44. Hamann J, Wishaupt JO, van Lier RA, Smeets TJ, Breedveld FC, Tak PP. Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 1999; 42:650–658.

45. Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018; 18:5–18.

46. Sogabe H, Nangaku M, Ishibashi Y, Wada T, Fujita T, Sun X, Miwa T, Madalo MP, Song WC. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001; 167:2791–2797.

47. Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, Cullen MJ, Richard SJ, Rollins SA, Mojcik CF, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004; 350:552–559.

48. Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003; 40:109–123.

49. Varsano S, Rashkovsky L, Shapiro H, Ophir D, Mark-Bentankur T. Human lung cancer cell lines express cell membrane complement inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clin Exp Immunol. 1998; 113:173–182.
50. Koretz K, Bruderlein S, Henne C, Moller P. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer. 1992; 66:810–814.

51. Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996; 149:129–142.
52. Inoue T, Yamakawa M, Takahashi T. Expression of complement regulating factors in gastric cancer cells. Mol Pathol. 2002; 55:193–199.

53. Ikeda J, Morii E, Liu Y, Qiu Y, Nakamichi N, Jokoji R, Miyoshi Y, Noguchi S, Aozasa K. Prognostic significance of CD55 expression in breast cancer. Clin Cancer Res. 2008; 14:4780–4786.

54. Cocco E, Varughese J, Buza N, Bellone S, Lin KY, Bellone M, Todeschini P, Silasi DA, Azodi M, Schwartz PE, et al. Tissue factor expression in ovarian cancer: implications for immunotherapy with hI-con1, a factor VII-IgGF(c) chimeric protein targeting tissue factor. Clin Exp Metastasis. 2011; 28:689–700.

55. Hara T, Kojima A, Fukuda H, Masaoka T, Fukumori Y, Matsumoto M, Seya T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol. 1992; 82:368–373.

56. Loberg RD, Day LL, Dunn R, Kalikin LM, Pienta KJ. Inhibition of decay-accelerating factor (CD55) attenuates prostate cancer growth and survival in vivo. Neoplasia. 2006; 8:69–78.
57. Durrant LG, Chapman MA, Buckley DJ, Spendlove I, Robins RA, Armitage NC. Enhanced expression of the complement regulatory protein CD55 predicts a poor prognosis in colorectal cancer patients. Cancer Immunol Immunother. 2003; 52:638–642.

58. Zell S, Geis N, Rutz R, Schultz S, Giese T, Kirschfink M. Down-regulation of CD55 and CD46 expression by anti-sense phosphorothioate oligonucleotides (S-ODNs) sensitizes tumour cells to complement attack. Clin Exp Immunol. 2007; 150:576–584.

59. Geis N, Zell S, Rutz R, Li W, Giese T, Mamidi S, Schultz S, Kirschfink M. Inhibition of membrane complement inhibitor expression (CD46, CD55, CD59) by siRNA sensitizes tumor cells to complement attack in vitro. Curr Cancer Drug Targets. 2010; 10:922–931.
60. Gao LJ, Guo SY, Cai YQ, Gu PQ, Su YJ, Gong H, Liu Y, Chen C. Cooperation of decay-accelerating factor and membrane cofactor protein in regulating survival of human cervical cancer cells. BMC Cancer. 2009; 9:384.

61. Gao LJ, Ding L, Guo SY, Cai YQ, Su YJ, Gong H, Liu Y, Chen C, Gu PQ. Role of decay-accelerating factor in regulating survival of human cervical cancer cells. J Cancer Res Clin Oncol. 2011; 137:81–87.

62. Hensel F, Timmermann W, von Rahden BH, Rosenwald A, Brandlein S, Illert B. Ten-year follow-up of a prospective trial for the targeted therapy of gastric cancer with the human monoclonal antibody PAT-SC1. Oncol Rep. 2014; 31:1059–1066.

63. Ward Y, Lake R, Martin PL, Killian K, Salerno P, Wang T, Meltzer P, Merino M, Cheng SY, Santoro M, et al. CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene. 2013; 32:2726–2738.

64. Liu D, Trojanowicz B, Ye L, Li C, Zhang L, Li X, Li G, Zheng Y, Chen L. The invasion and metastasis promotion role of CD97 small isoform in gastric carcinoma. PLoS One. 2012; 7:e39989.

65. Wobus M, Huber O, Hamann J, Aust G. CD97 overexpression in tumor cells at the invasion front in colorectal cancer (CC) is independently regulated of the canonical Wnt pathway. Mol Carcinog. 2006; 45:881–886.

66. Andoh A, Fujiyama Y, Sumiyoshi K, Sakumoto H, Bamba T. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology. 1996; 111:911–918.

67. Nasu J, Mizuno M, Uesu T, Takeuchi K, Inaba T, Ohya S, Kawada M, Shimo K, Okada H, Fujita T, et al. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin Exp Immunol. 1998; 113:379–385.
68. Spiller OB, Criado-Garcia O, Rodriguez De Cordoba S, Morgan BP. Cytokine-mediated up-regulation of CD55 and CD59 protects human hepatoma cells from complement attack. Clin Exp Immunol. 2000; 121:234–241.

69. Takeuchi K, Mizuno M, Uesu T, Nasu J, Kawada M, Hori S, Okada H, Endo Y, Fujita T, Tsuji T. Epidermal growth factor induces expression of decay-accelerating factor in human colonic cancer cells via the mitogen-activated protein kinase pathway. J Lab Clin Med. 2001; 138:186–192.

70. Li L, Spendlove I, Morgan J, Durrant LG. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001; 84:80–86.

71. Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, Amadori A, Tedesco F. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007; 67:10556–10563.
72. Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol. 2013; 7:580–594.

73. Gorter A, Blok VT, Haasnoot WH, Ensink NG, Daha MR, Fleuren GJ. Expression of CD46, CD55, and CD59 on renal tumor cell lines and their role in preventing complement-mediated tumor cell lysis. Lab Invest. 1996; 74:1039–1049.
74. Madjd Z, Durrant LG, Bradley R, Spendlove I, Ellis IO, Pinder SE. Loss of CD55 is associated with aggressive breast tumors. Clin Cancer Res. 2004; 10:2797–2803.

75. Bergelson JM, Chan M, Solomon KR, St John NF, Lin H, Finberg RW. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci U S A. 1994; 91:6245–6248.

77. Biryukov S, Stoute JA. Complement activation in malaria: friend or foe? Trends Mol Med. 2014; 20:293–301.

78. Tham WH, Kennedy AT. Malaria: a master lock for deadly parasites. Nature. 2015; 522:158–159.
79. Angeletti A, Marasà M, Cravedi P. CD55 deficiency and protein-losing enteropathy. N Engl J Med. 2017; 377:1499.

81. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333:1253–1258.

82. Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008; 22:65–74.

83. Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008; 180:5882–5889.
84. Li Q, Huang D, Nacion K, Bu H, Lin F. Augmenting DAF levels in vivo ameliorates experimental autoimmune encephalomyelitis. Mol Immunol. 2009; 46:2885–2891.
85. Karpus ON, Kiener HP, Niederreiter B, Yilmaz-Elis AS, van der Kaa J, Ramaglia V, Arens R, Smolen JS, Botto M, Tak PP, et al. CD55 deposited on synovial collagen fibers protects from immune complex-mediated arthritis. Arthritis Res Ther. 2015; 17:6.

86. Hoek RM, de Launay D, Kop EN, Yilmaz-Elis AS, Lin F, Reedquist KA, Verbeek JS, Medof ME, Tak PP, Hamann J. Deletion of either CD55 or CD97 ameliorates arthritis in mouse models. Arthritis Rheum. 2010; 62:1036–1042.

87. Le Poole IC, Luiten RM. Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. 2008; 10:227–243.

88. van den Wijngaard RM, Asghar SS, Pijnenborg AC, Tigges AJ, Westerhof W, Das PK. Aberrant expression of complement regulatory proteins, membrane cofactor protein and decay accelerating factor, in the involved epidermis of patients with vitiligo. Br J Dermatol. 2002; 146:80–87.

89. Miwa T, Maldonado MA, Zhou L, Yamada K, Gilkeson GS, Eisenberg RA, Song WC. Decay-accelerating factor ameliorates systemic autoimmune disease in MRL/lpr mice via both complement-dependent and -independent mechanisms. Am J Pathol. 2007; 170:1258–1266.

90. Moran P, Beasley H, Gorrell A, Martin E, Gribling P, Fuchs H, Gillett N, Burton LE, Caras IW. Human recombinant soluble decay accelerating factor inhibits complement activation in vitro and in vivo. J Immunol. 1992; 149:1736–1743.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download