INTRODUCTION
The coronavirus disease 2019 (COVID-19) vaccination campaign is globally underway to curb the spread of COVID-19, which has affected over 1.8 billion people globally.
1 In South Korea, the COVID-19 vaccines, developed by AstraZeneca and Pfizer–BioNTech, have been rolled out with priority for long-term care facility residents and healthcare workers since February 26, 2021.
2 Given that older people are at greater risk of severe COVID-19,
34 individuals who are ≥ 75 years of age are the next priority group for COVID-19 vaccination among the general population, and the vaccination campaign for them was started in the first week of April, 2021. However, older adults, especially those ≥ 75 years of age, are usually not included in novel vaccine clinical trials and tend to have several underlying diseases; consequently, there have been concerns about administering COVID-19 vaccination to them.
5 Therefore, this study aimed to investigate the safety of the COVID-19 vaccine for people aged ≥ 75 years, specifically those who first completed the 2-dose vaccine schedule in South Korea.
METHODS
Study design and participants
The COVID-19 central vaccination center—run by the National Medical Center, which is located in Jung-gu, Seoul, South Korea—started vaccinating people aged ≥ 75 years on April 5, 2021. The vaccinees were those who lived in the same district as the vaccination center and could visit the vaccination center by themselves or with a caregiver's assistance. The Pfizer–BioNTech BNT162b2 COVID-19 vaccine was used according to the national vaccination policy. This study included participants who received the first dose of the BNT162b2 vaccine between April 5 and April 23 and completed the second dose between April 26 to May 14, 2021.
Safety monitoring after COVID-19 vaccination was carried out in three ways for the following period and methods: 1) active monitoring for immediate reactions after the injection for all vaccine recipients, 2) active monitoring of solicited and unsolicited adverse events after 7 days following each dose for selected vaccine recipients, and 3) passive surveillance of medically attended adverse events (MAAEs) for all vaccine recipients from the day of the first dose until 2 weeks after the second dose (
Fig. 1). For immediate adverse reactions, every person who was vaccinated was observed for either 15 or 30 minutes at the center, depending on the previous history of allergic reactions of each person.
Fig. 1
Study scheme.
AE = adverse event, MAAE = medically attended adverse event, KDCA = Korea Disease Control and Prevention Agency.
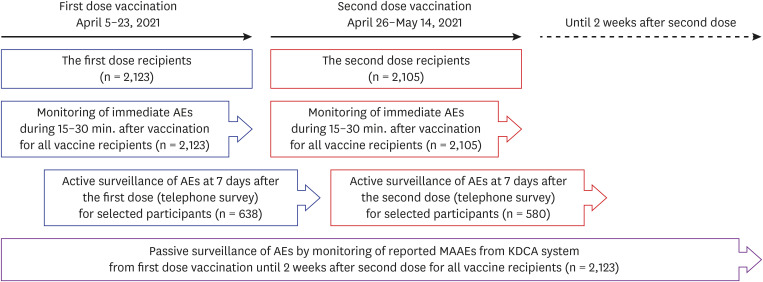

Active surveillance was conducted for selected people who agreed to provide information regarding the occurrence of adverse reactions after vaccination, using stratified random sampling. A subset of selected people was followed up by telephone interview 7 days after each vaccination. The interview respondent was either the person vaccinated, or a representative who lived with and was well aware of the condition of the person. We asked whether the vaccinated person experienced any reaction and enquired about severity, onset, and duration of symptoms related to the reaction if there were affirmative answers. People who did not respond to telephone interviews were called again within a day until a maximum of three contact attempts were made.
For passive surveillance of adverse events among study participants, we collected data of the COVID-19 vaccine adverse event following immunization (AEFI) surveillance system, which was obtained from the Korea Disease Control and Prevention Agency (KDCA). We reviewed all cases of MAAEs reported among study participants between the day of the first dose and 2 weeks after the second dose.
Data collection and outcomes
Through telephone interviews, we collected information on the sex, age, use of antipyretics or analgesics, and adverse reactions. The survey questionnaire included questions on occurrence and severity grade of any local (pain, redness, or swelling at the vaccination site) and systemic (fever, chills, headache, fatigue, muscle pain, joint pain, nausea and/or vomiting, diarrhea) adverse events for 7 days post vaccination. We graded adverse events as grade 1 (mild) for no interference with activity, grade 2 (moderate) for some interference with activity, grade 3 (severe) for significant and preventing daily activities, and grade 4, if necessitating emergency room (ER) visit or hospitalization. To evaluate the occurrence and severity of fever, we first enquired whether the vaccinated person had a fever or felt feverish. If the answer was affirmative, the peak temperature, if measured, and the degree to which the symptoms interfered with the daily life of the person were noted. The questionnaire sheet used in the interview is shown in
Supplementary Data 1.
Statistical analyses
We did not determine the number of interviewees on the basis of a statistical power calculation but attempted as many telephone interviews as possible from a stratified random sample of people willing to answer the questionnaire.
Statistical analyses were conducted using R 4.0 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were performed for the collected data. The responses were displayed as frequency (counts and/or percentage). Proportions of adverse reactions were compared between age groups by use of the Cochran–Armitage trend analysis. The age groups were classified into 5-year intervals as follows: 75–79, 80–84, and 85 years or older. Proportions of adverse reactions between dose or sex were compared using of χ2 or Fisher's exact test. A P value < 0.05 was considered statistically significant.
Ethics statement
The National Medical Center Institutional Review Board approved this study (No. NMC-2021-04-056) and need for written consent was waived.
RESULTS
During the study period, 2,123 individuals aged ≥ 75 years received the first dose of the BNT162b2 vaccine. At 21 days from the first dose, 97.3% of them received the second dose. Eighteen people (0.8%) did not complete the second dose within 6 weeks after the first dose.
Demographics
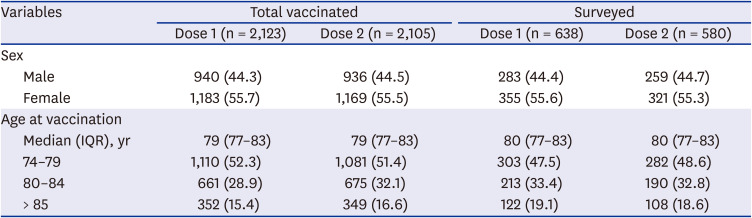
Of the 2,123 vaccinated individuals, 44.4% were male. The median age of the individuals was 79 years (range 75–102 years). Of the 638 people who responded to active surveillance, 44.4% were male and the median age was 80 years (range 75–97 years) (
Table 1).
Table 1
Demographic data of all vaccinated and surveyed individuals
|
Variables |
Total vaccinated |
Surveyed |
|
Dose 1 (n = 2,123) |
Dose 2 (n = 2,105) |
Dose 1 (n = 638) |
Dose 2 (n = 580) |
|
Sex |
|
|
|
|
|
Male |
940 (44.3) |
936 (44.5) |
283 (44.4) |
259 (44.7) |
|
Female |
1,183 (55.7) |
1,169 (55.5) |
355 (55.6) |
321 (55.3) |
|
Age at vaccination |
|
|
|
|
|
Median (IQR), yr |
79 (77–83) |
79 (77–83) |
80 (77–83) |
80 (77–83) |
|
74–79 |
1,110 (52.3) |
1,081 (51.4) |
303 (47.5) |
282 (48.6) |
|
80–84 |
661 (28.9) |
675 (32.1) |
213 (33.4) |
190 (32.8) |
|
> 85 |
352 (15.4) |
349 (16.6) |
122 (19.1) |
108 (18.6) |

Adverse reactions occurring immediately after vaccination
Of the 2,123 individuals who received the first dose, 57 reported a history of allergic reactions. Except for three individuals with histories of specific drug-related angioedema, the rest were mild allergic reactions. There were 36 cases (22 after the first dose and 14 after the second dose) with adverse reactions during 15 or 30 minutes after injection (8.5 cases per 1,000 dose). Common symptoms were dizziness (n = 16, 41.7%), headache (n = 9, 25.0%), nausea (n = 4, 11.1%), and chest discomfort and/or dyspnea (n = 4, 11.1%). Although none of the reactions was assessed as allergic reaction to the vaccine, immediate reactions occurred more frequently in individuals with a previous history of allergic reactions (P < 0.001). The median onset of symptoms was 18 minutes (range, 5–36 minutes) from the injection. There were no cases that required special treatment or a drug to be administered, and the symptoms resolved or improved after bed rest. Among these 36 cases, there were no cases that were reported to the KDCA for visiting a hospital after returning home.
Active surveillance of adverse events within 7 days
Among the 2,123 individuals who received the first dose, we attempted to contact 807 (38.0% of total vaccinated) via telephone interviews for active surveillance. 638 (30.1% of total vaccinated) individuals responded with a response rate of 79.1%. Those who responded to the first survey were interviewed after the second dose. Five-hundred and eighty people were interviewed in the second survey with a response rate of 90.9%, and 58 were included only in the first survey. The overall self-response rate was 82.3%: 89.2% in those aged 75–79 years, 79.7% in those aged 80–84 years, and 69.1% in those aged ≥ 85 years. The older the age group, the higher was the rate of proxy responses (P < 0.001). In each age group (75–79, 80–84, and ≥ 85 years), the proxy response did not have a statistically significant effect on the rate of each adverse reaction.
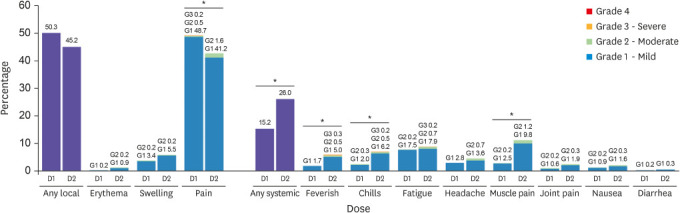
The frequency, grade, onset, and duration of adverse reactions after each dose of the vaccine were compared in
Fig. 2 and
Table 2. In general, adverse reactions were mild-to-moderate in severity, began on day 1 or 2 after vaccination, and mostly resolved within 3 days. The rates of local reactions were 50.3% after the first dose and 45.2% after the second dose. The rates of overall local reactions and injection site pain did not increase after the second dose. Although the reported frequency of injection site erythema, swelling, and itching increased after the second dose, the differences were not statistically significant. The rates of systemic reactions were 15.2% after the first dose and 26.0% after the second dose. For systemic reactions, particularly the frequencies of sense of fever, chills, and muscle pain were found to be significantly higher after the second dose. Among 34 individuals who felt feverish, only 5 had a fever of 37.5°C or higher, measured with a thermometer. Two individuals had low-grade fever between 37.5–38.0°C after the first dose and three had fever with the maximum body temperature of 37.8°C, 38.0°C, and 39.0°C, respectively after the second dose.
Fig. 2
Frequencies and grades of adverse reactions after each dose of the vaccine. The percentages of participants reporting any solicited reaction and specific solicited reactions are shown. Results included all participants who responded to the active monitoring. The “Any” category shows the overall percentage of participants reporting any solicited injection site or systemic reaction. Pain at the injection site was assessed according to the following scale: mild, does not interfere with activity; moderate, interferes with activity; severe, prevents daily activity; and grade 4, emergency room visit or hospitalization. Redness and swelling were measured according to the following scale: mild, 2.0 to 5.0 cm in diameter; moderate, > 5.0 to 10.0 cm in diameter; severe, > 10.0 cm in diameter; and grade 4, necrosis or exfoliative dermatitis (for redness) and necrosis (for swelling). Scales used for most systemic adverse reactions were as follows: feverish, chills, fatigue, headache, muscle pain, joint pain (mild: does not interfere with activity; moderate: some interference with activity; or severe: prevents daily activity), nausea and/or vomiting (mild: 1 to 2 times in 24 hours; moderate: > 2 times in 24 hours; or severe: requires intravenous hydration), and diarrhea (mild: 2 to 3 loose stools in 24 hours; moderate: 4 to 5 loose stools in 24 hours; or severe: 6 or more loose stools in 24 hours); grade 4 for all reactions indicated an ER visit or hospitalization.
*Indicates P value < 0.05.
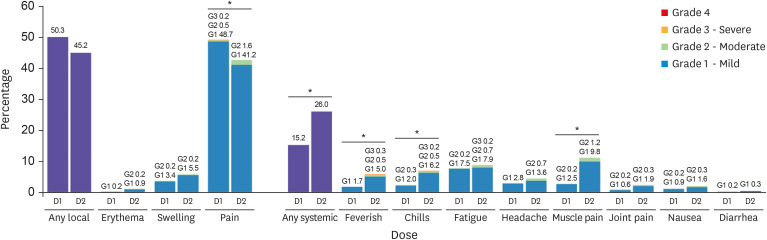

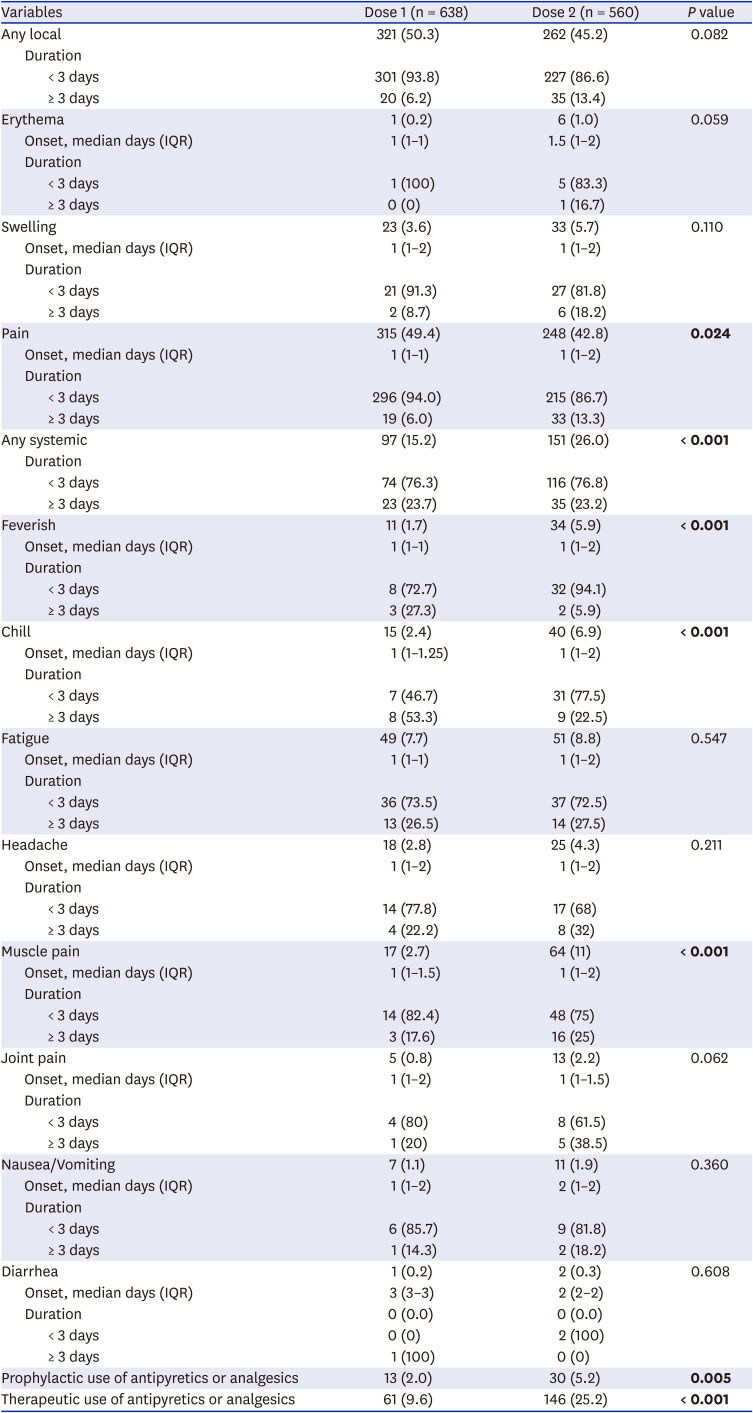
Table 2
Active surveillance results for local and systemic adverse reactions following each dose
|
Variables |
Dose 1 (n = 638) |
Dose 2 (n = 560) |
P value |
|
Any local |
321 (50.3) |
262 (45.2) |
0.082 |
|
Duration |
|
|
|
|
< 3 days |
301 (93.8) |
227 (86.6) |
|
|
≥ 3 days |
20 (6.2) |
35 (13.4) |
|
Erythema |
1 (0.2) |
6 (1.0) |
0.059 |
|
Onset, median days (IQR) |
1 (1–1) |
1.5 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
1 (100) |
5 (83.3) |
|
|
≥ 3 days |
0 (0) |
1 (16.7) |
|
Swelling |
23 (3.6) |
33 (5.7) |
0.110 |
|
Onset, median days (IQR) |
1 (1–2) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
21 (91.3) |
27 (81.8) |
|
|
≥ 3 days |
2 (8.7) |
6 (18.2) |
|
Pain |
315 (49.4) |
248 (42.8) |
0.024
|
|
Onset, median days (IQR) |
1 (1–1) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
296 (94.0) |
215 (86.7) |
|
|
≥ 3 days |
19 (6.0) |
33 (13.3) |
|
Any systemic |
97 (15.2) |
151 (26.0) |
< 0.001
|
|
Duration |
|
|
|
|
< 3 days |
74 (76.3) |
116 (76.8) |
|
|
≥ 3 days |
23 (23.7) |
35 (23.2) |
|
Feverish |
11 (1.7) |
34 (5.9) |
< 0.001
|
|
Onset, median days (IQR) |
1 (1–1) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
8 (72.7) |
32 (94.1) |
|
|
≥ 3 days |
3 (27.3) |
2 (5.9) |
|
Chill |
15 (2.4) |
40 (6.9) |
< 0.001
|
|
Onset, median days (IQR) |
1 (1–1.25) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
7 (46.7) |
31 (77.5) |
|
|
≥ 3 days |
8 (53.3) |
9 (22.5) |
|
Fatigue |
49 (7.7) |
51 (8.8) |
0.547 |
|
Onset, median days (IQR) |
1 (1–1) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
36 (73.5) |
37 (72.5) |
|
|
≥ 3 days |
13 (26.5) |
14 (27.5) |
|
Headache |
18 (2.8) |
25 (4.3) |
0.211 |
|
Onset, median days (IQR) |
1 (1–2) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
14 (77.8) |
17 (68) |
|
|
≥ 3 days |
4 (22.2) |
8 (32) |
|
Muscle pain |
17 (2.7) |
64 (11) |
< 0.001
|
|
Onset, median days (IQR) |
1 (1–1.5) |
1 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
14 (82.4) |
48 (75) |
|
|
≥ 3 days |
3 (17.6) |
16 (25) |
|
Joint pain |
5 (0.8) |
13 (2.2) |
0.062 |
|
Onset, median days (IQR) |
1 (1–2) |
1 (1–1.5) |
|
Duration |
|
|
|
|
< 3 days |
4 (80) |
8 (61.5) |
|
|
≥ 3 days |
1 (20) |
5 (38.5) |
|
Nausea/Vomiting |
7 (1.1) |
11 (1.9) |
0.360 |
|
Onset, median days (IQR) |
1 (1–2) |
2 (1–2) |
|
Duration |
|
|
|
|
< 3 days |
6 (85.7) |
9 (81.8) |
|
|
≥ 3 days |
1 (14.3) |
2 (18.2) |
|
Diarrhea |
1 (0.2) |
2 (0.3) |
0.608 |
|
Onset, median days (IQR) |
3 (3–3) |
2 (2–2) |
|
Duration |
0 (0.0) |
0 (0.0) |
|
|
< 3 days |
0 (0) |
2 (100) |
|
|
≥ 3 days |
1 (100) |
0 (0) |
|
Prophylactic use of antipyretics or analgesics |
13 (2.0) |
30 (5.2) |
0.005
|
|
Therapeutic use of antipyretics or analgesics |
61 (9.6) |
146 (25.2) |
< 0.001
|

While most of the reactions were mild after the second dose, there was an increase in the proportion of individuals with grade 2 or higher reactions (1.3% after dose 1, 4.0% after dose 2, P = 0.005). There was one grade 4 reaction that required an ER visit. In this case, the vaccine recipient was a woman in her 90s and she visited the ER due to chest discomfort on the day of the second dose but did not require hospitalization or special treatment. A total of 9.6% after the first dose and 25.2% after the second dose used antipyretics or analgesics for post-vaccination symptoms. There was no unusual or serious adverse reaction that was found through this active monitoring by telephone interview.
Most adverse reactions were more common in the age group of 75–79 years. In both the first and the second doses, there were trends of lower rates of pain at the vaccination site (
P < 0.001,
P = 0.024) and overall systemic reactions (
P = 0.008,
P = 0.028) in the older group. Also, fewer people in the older group had sense of fever or muscle pain after the second dose (
P = 0.042,
P = 0.002) (
Supplementary Table 1 and
Supplementary Fig. 1).
When comparing the prevalence of adverse reactions according to sex, the prevalence of most symptoms was higher among women than among men (
Supplementary Table 2). Specifically, more women than men had overall systemic adverse reactions after each dose (
P < 0.001,
P = 0.038), and fatigue (
P = 0.013,
P = 0.032).
Passive surveillance of MAAEs
Of the 4,228 doses of vaccine administered, there were 23 medically attended adverse cases (5.4 cases per 1,000 administered doses) that were reported by physicians through the KDCA AEFI surveillance system. Overall, five serious adverse events (SAEs), which required hospitalization, were reported including one death, and the rest of the eighteen cases were reported as those requiring a visit to the ER or outpatient department/clinic. The final diagnoses of the SAEs were hepatic encephalopathy with sepsis (one case of death), epidural abscess, Bell's palsy, acute cerebral infarction, and duodenal ulcer (
Supplementary Table 3). The deceased patient had liver cirrhosis with a history of recurrent encephalopathy and expired due to sepsis progression 4 days after admission. Other patients with SAE, except the one with epidural abscess, were discharged after symptoms were relieved.
The most common reasons for visiting the ER or outpatient department were nonspecific general weakness (26%) and dizziness (26%), followed by muscle pain (22%), headache (13%), fever (13%), and skin rash/urticaria (13%) (
Table 3). MAAE reporting rates (cases per 1,000 administered doses) were higher in the older group and females than that in the younger group and males. In particular, people aged ≥ 85 years visited health care facilities for adverse events two times more than people aged 75–79 years. Two thirds of the individuals with MAAEs visited health care facilities within 3 days after vaccination. There were only three allergic reaction cases of reported skin rash or urticaria, and all of them visited the ER after the second dose of vaccine. One patient had prolonged pain in the injection-side arm and shoulder for over 2 weeks, which was suspected to be due to a shoulder joint injury related to vaccine administration.
Table 3
Characteristics of the reported medically attended adverse events
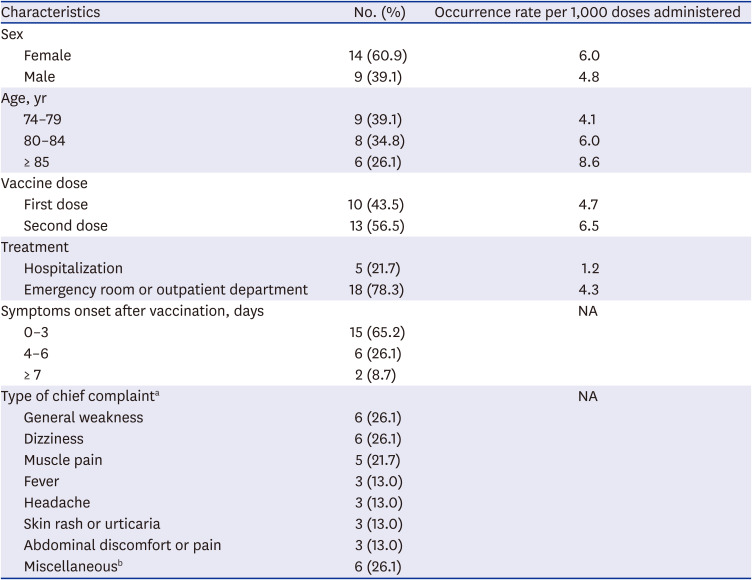
|
Characteristics |
No. (%) |
Occurrence rate per 1,000 doses administered |
|
Sex |
|
|
|
Female |
14 (60.9) |
6.0 |
|
Male |
9 (39.1) |
4.8 |
|
Age, yr |
|
|
|
74–79 |
9 (39.1) |
4.1 |
|
80–84 |
8 (34.8) |
6.0 |
|
≥ 85 |
6 (26.1) |
8.6 |
|
Vaccine dose |
|
|
|
First dose |
10 (43.5) |
4.7 |
|
Second dose |
13 (56.5) |
6.5 |
|
Treatment |
|
|
|
Hospitalization |
5 (21.7) |
1.2 |
|
Emergency room or outpatient department |
18 (78.3) |
4.3 |
|
Symptoms onset after vaccination, days |
|
NA |
|
0–3 |
15 (65.2) |
|
4–6 |
6 (26.1) |
|
≥ 7 |
2 (8.7) |
|
Type of chief complainta
|
|
NA |
|
General weakness |
6 (26.1) |
|
Dizziness |
6 (26.1) |
|
Muscle pain |
5 (21.7) |
|
Fever |
3 (13.0) |
|
Headache |
3 (13.0) |
|
Skin rash or urticaria |
3 (13.0) |
|
Abdominal discomfort or pain |
3 (13.0) |
|
Miscellaneousb
|
6 (26.1) |

DISCUSSION
Older people are considered to have high priority for COVID-19 vaccines
6; however, little is known about the safety of vaccines in them. Our study showed that people aged ≥ 75 years reported an overall 50% and 45% local adverse events and 15% and 26% systemic adverse events for the first and the second dose of the vaccine, respectively. Most of them had mild reactogenicity events and recovered within 3 days. There were no SAEs or clustered cases found that were possibly related to the COVID-19 vaccine from the reviews of reported MAAEs. Overall, the BNT162b2 vaccine is considered safe for older adults with multiple comorbidities. To the best of our knowledge, this is the first study monitoring the safety of COVID-19 vaccines for people aged ≥ 75 years in South Korea.
Adverse events after vaccination were noticeably less frequent in the respondents included in our study than those included in prior studies,
78 although the types of common adverse events after vaccination were similar. In the phase 2/3 clinical trial of the BNT162b2 vaccine,
7 among about 8,000 participants who were older than 55 years of age, pain at the injection site was reported in 71% and 66%, followed by fatigue in 34% and 51%, headache in 25% and 39%, muscle pain in 14% and 29%, and joint pain in 9% and 19% after the first and second dose of the BNTb16b2 vaccine, respectively. Early safety monitoring of health care workers, as well as clinical trials, consistently showed that younger people experienced more adverse reactions after BNT162b2 vaccination than the older people.
91011 The differences in the frequency of adverse events between our study and prior studies might be from the proportion of super-agers. In a study by Polack et al.,
7 people aged over 75 years accounted for only 10% of their study population.
12 In contrast, Menni et al.
13 found that adverse reactions in a real-world community setting were much less common than in phase 3 clinical trials. Another recent study
14 compared the immunogenicity and reactogenicity between people aged < 60 years and > 80 years, and showed that 94% and 83% of the people aged > 80 years reported no reaction after the first dose and second dose of vaccination, respectively. Older people may be less likely to experience adverse reactions than younger people in clinical trials,
78 although a further study in a larger population is needed. In addition, the timing at which the telephone survey was conducted, 7 days after vaccination, could affect the results. Since they had already recovered from adverse reactions, vaccine recipients may tend to underreport their reactions.
Except for the incidence of adverse reactions, our results were in line with prior studies.
789 Compared with the first dose of the vaccine, more people reported systemic adverse reactions, and the severity of systemic adverse events also increased in the second dose. This is probably because the subsequent dose provoked a more robust immune response since the immunity to viral antigens had already developed after the first dose. Likewise, vaccine recipients with a history of COVID-19 report more adverse reactions than those without a history of COVID-19.
13 In addition, older people and males reported fewer adverse reactions than the relatively younger people and females. Sex differences in reported vaccine adverse reactions were common in COVID-19 vaccines
91015 as well as other vaccines.
1617 While a few explanations attribute this phenomenon to hormonal differences, the sex differences to vaccine responses are generally considered as multifactorial with both biological and behavioral factors.
1618 Furthermore, considering that sex hormone differences between men and women decreases due to aging,
19 the differences by sex in the levels of perception of subjective discomfort or differences in the tendency to report discomfort may also affect the observed sex difference in our study.
In contrast, no acute allergic response was observed for the 4,228 doses administered during the study period at our vaccine center. Given that anaphylaxis is very rare and 4.5 cases were reported per million vaccine doses administered,
20 anaphylaxis was not expected in the small number of vaccine recipients in this study. Moreover, other acute allergic or anxiety-related reactions for which medications or referring to the ER were needed did not occur either. There was a relatively lower incidence of acute reactions in young healthcare workers who were vaccinated at our center; among them, 21 (0.2%) of 10,510 doses needed medication for acute symptoms right after vaccination and 5 cases (0.05%) were referred to the ER for various acute symptoms.
We also reviewed MAAEs reported through AEFI surveillance system. Overall, 5.4 MAAEs per 1,000 administered doses were reported for 4,228 administered doses, including the first and the second dose. According to the National AEFI surveillance of the BNTb162b vaccine for the first month, 4.2 MAAEs per 1,000 administered doses were reported.
21 Given that young healthcare workers were the first recipients of the BNTb162b vaccine for the first month, our MAAE reporting rate seems slightly higher. This may be attributed not only to the high proportion of comorbidity in the oldest vaccine recipients but also to the high reporting rate by the physicians at our institution. We had promoted and educated reporting of AEFI to frontline physicians before the COVID-19 vaccine center was launched. By contrast, most of the MAAEs were not serious and were mostly nonspecific general weakness and dizziness. Among 5 SAEs reported, Bell's palsy and stroke are in the list of adverse events of special interest (AESIs) defined by United States Food and Drug Administration.
22 The incidence of Bell's palsy was higher in the vaccine group (4 cases) compared with the placebo group (0 cases) in the phase 3 clinical trial.
7 Ozonoff and colleagues
23 concluded that the observed incidence of Bell's palsy in the mRNA vaccine arms was 3.5 to 7 times higher than expected in the general population through data inferred from mRNA vaccine clinical trials. In contrast, a recent case-control study by Shemer et al.
24 did not show any association of Bell's palsy with the BNT162b2 vaccine. In addition, any relevant signal regarding Bell's palsy or stroke after BNTb162b vaccination was not presented through vaccine safety monitoring system underway globally in the real-world vaccination campaign.
2526 The other SAEs were due to other diseases rather than the vaccine; hence, their causality cannot be related to the vaccine. Further monitoring of relevant adverse events for a larger population is needed for the confirmation of the causal relationship of the proposed AESI.
There are several limitations to this study. First, since we conducted the telephone survey 7 days after vaccination, the frequency and severity of adverse events could be underestimated as symptoms would have lessened. Further, it is possible to misunderstand older people, or they may answer incorrectly, particularly during a telephone interview due to lack of direct communication. Interviewers' attitudes or how the questions are asked could influence the respondents' answers, although we had educated the interviewers before the survey. Second, the total number of vaccine recipients and the observed time after vaccination were not enough to conclude the safety of the BNT162b2 vaccine. It is impossible to find very rare adverse reactions and signals. However, we surveyed as many as 30% of vaccine recipients, which could be a sufficient representation of the total vaccine recipients for monitoring solicited reactions. Lastly, we did not investigate the underlying diseases or comorbidities of study participants. Moreover, since we included the proxy response as well as the self-response for the survey, the proxy answer could provide a bias for outcomes particularly in older people over 85 years. However, the results were not significantly different whether proxy responses were included or not. Furthermore, including proxy responses could be the strength of our study considering the monitoring of adverse reactions in older adults, who could not be examined by other studies.
In conclusion, the BNT162b2 vaccination was safe and tolerable in the adults aged ≥ 75 years. The need for COVID-19 vaccine booster shots has been raised. According to Israel's data, the safety profile of the booster dose appears to be similar to that of the first and second doses.
27 The results of our study can provide valuable information to vaccine providers for counseling older adults about what to expect after vaccination and public health authorities while planning COVID-19 vaccination campaigns for older adults. Further monitoring of relevant adverse events including proposed AESIs for a larger population over a longer period is needed to confirm the safety of the COVID-19 vaccine.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download