This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Regular assessments of clinical performance in gynecologic cancer surgery is important for the safety of patients. We evaluated the effects of quality control (QC) program on the treatment pattern and clinical outcomes of early cervical cancer.
Methods
Medical records of cervical cancer patients who received operation in our institution from January 2007 to December 2018 were retrospectively reviewed. Cases were divided into 2 groups, before and after the initiation of QC program, group 1 (2007–2013) and group 2 (2014–2018), based on the operation date. Two groups were compared in clinicopathologic variables, surgical methods, operative details, adjuvant treatments, recurrence and survival.
Results
A total of 305 cervical cancer patients were included in the analysis, 210 in group 1 and 95 in group 2. In group 2, minimally invasive surgery (MIS) was more frequently performed (60.0% vs. 76.8%, P = 0.004), especially in earlier stages (stage IA, 72.6% vs. 100.0%; stage IB, 52.2% vs. 69.5%). However, the median tumor size treated by MIS was decreased in stage IB (20 mm vs. 17 mm, P = 0.015). Frequency of adjuvant treatment was also reduced in stage IB (56.5% vs. 37.3%, P = 0.016). Recurrence within 3 years, 3-year disease free survival and overall survival did not show significant difference; however, 3-year recurrence after MIS was significantly reduced in stage IB.
Conclusion
QC program enforced stricter patient selection criteria for MIS and positively affected clinical outcomes in cervical cancer patients who underwent surgery. Systemic monitoring should be considered for patient safety.
Keywords: Quality Control, Uterine Cervical Neoplasms, Gynecologic Surgical Procedures, Hysterectomy
INTRODUCTION
Mainstays of treatment of early stage cervical cancer (International Federation of Gynecology and Obstetrics [FIGO] stages IA1 through IIA), is surgery for medically suitable patients.
1 Primary goal of surgery in cervical cancer, as with any other types of cancers, is adequate removal of the tumor with sufficient resection margin, as well as exploration and excision of possible sites of metastasis through lymphadenectomy in appropriate cases, while minimizing complications. It has been clear, that the quality and extent of surgery influence the perioperative complications, overall morbidity, local tumor control and eventually, recurrence and survival.
234 In other gynecologic cancers including ovarian cancer, it has been proved that the clinical outcome was superior when treated in quality controlled, specialized institution, by specialists.
35 This is possibly due to appropriate preoperative judgment, accurate diagnosis, optimal surgical technique as well as effective postoperative care. Centers with higher volume of patients and physicians of specialty have also been associated with positive clinical outcomes.
6 Thus, importance of quality control (QC) and assessment has increasingly become of concern.
Recent publication of Laparoscopic Approach to Cervical Cancer (LACC) trial in 2018 suggested inferior survival with MIS compared to open surgery in early cervical cancer.
7 This cornerstone trial sparked heated debates on surgical techniques and proficiency of MIS performed by different surgeons, as well as challenged current notions of oncologic equivalency of MIS with abdominal method of radical hysterectomy.
8910 Many suggestions and studies have been published regarding surgical techniques and precautions,
811 but one should note that such QC is not a sole share of the operator; intervention and quality monitoring should be done on institutional level.
In our institution, we realized optimal care for cancer patients involve co-work of multidisciplinary team, and found the need to intervene by holding regular QC meetings that involve every member of the department for quality monitoring. Before such QC meetings started in 2014, treatment plans and methods were decided by individual surgeon on his or her discretion. After departmental level intervention in cancer patient management flow has been implemented, active feedback on management plans were provided monthly in a form of QC meeting to standardize treatment and reduce the heterogeneity among surgeons.
In this study, we evaluated how the implementation of quality monitoring sessions affected surgeons' decision in treating early stage cervical cancer, and ultimately cervical cancer patients' oncologic outcomes.
METHODS
The medical records of cervical cancer patients who received operation in Ewha Womans University Mokdong Hospital from January 2007 to December 2018, were retrospectively reviewed.
The inclusion criteria were as follows: 1) histologically confirmed cervical cancer; 2) preoperative FIGO stage IA-IIA2; 3) who received operation (type A-C2 hysterectomy or trachelectomy ± lymphadenectomy) as a primary treatment. The exclusion criteria were as follows: 1) patients who underwent hysterectomy for other reasons, but was incidentally found to have invasive cervical cancer; 2) patients who received neoadjuvant chemotherapy with or without radiotherapy.
The quality assessment meetings started in year 2014, as part of departmental QC measures to better monitor cancer patient management. All cancer patients were included as subjects of QC meetings, and all the members of the department, including gynecologic oncologists, related specialists as well as trainees, participated. Individual patient cases were discussed in detail, and their management flow of initial workup, perioperative management, operation method, pathologic results, adjuvant treatment and follow-up were thoroughly discussed among participants, and active feedback was provided monthly. Example of a recording form used during QC meetings is included as
Supplementary Fig. 1.
Since the QC measure was implemented in 2014, the patients were divided into 2 period groups (group 1, 2007-2013 and group 2, 2014-2018), based on their date of operation. Baseline patient characteristics and treatment details were collected, which included age at diagnosis, stage, surgical methods (open vs. minimally invasive methods – robotic or laparoscopic), operation time, estimated blood loss, intraoperative and postoperative complications, days of hospital stay, and adjuvant treatments. Pathologic outcomes, such as histologic type, tumor size, depth of stromal invasion, lymphovascular invasion, lymph node metastasis, parametrial invasion and surgical margin were also collected. FIGO staging system of cervical cancer was revised in 2018 during the study period, thus we reassigned the stage of all cases into 2018 FIGO staging for study analysis. Recurrence was confirmed pathologically by biopsy or cytology, or radiologically with computer tomography (CT), magnetic resonance imaging or position emission tomography-CT (PET-CT). The date of recurrence was determined by date of first exam performed with such findings.
In order to compare clinical outcomes of 2 groups from different time periods without bias that could result from longer follow-up duration of group 1, recurrence within 3 years of follow-up were compared between the two groups. 3-year recurrence and 3-year disease free survival were calculated from the date of operation. Disease free survival was calculated from the date of operation to the date of recurrence or death due to cervical cancer. For the patients with no evidence of recurrence or death, their last date of follow-up was censored at their last date of visit at our outpatient clinic.
Statistical analysis was performed using IBM SPSS software for Windows (version 21.0; SPSS Inc., Chicago, IL, USA). To compare clinicopathologic characteristics and operation methods between group 1 and 2, independent sample t test was used for continuous variables with confirmed normal distribution. For variables that are not normally distributed or are categorical, χ2 test or nonparametric tests were used. Survival curves were generated using Kaplan-Meier method, and compared between the two groups using log-rank test. 3-year recurrence and adjuvant treatment of each stage in group 1 and 2 were analyzed using χ2 test. The P value of < 0.05 was considered statistically significant.
Ethics statement
The approval of waiver of informed consent from Institutional Review Board (IRB No. EUMC 2014-12-005-001) was received prior to study.
RESULTS
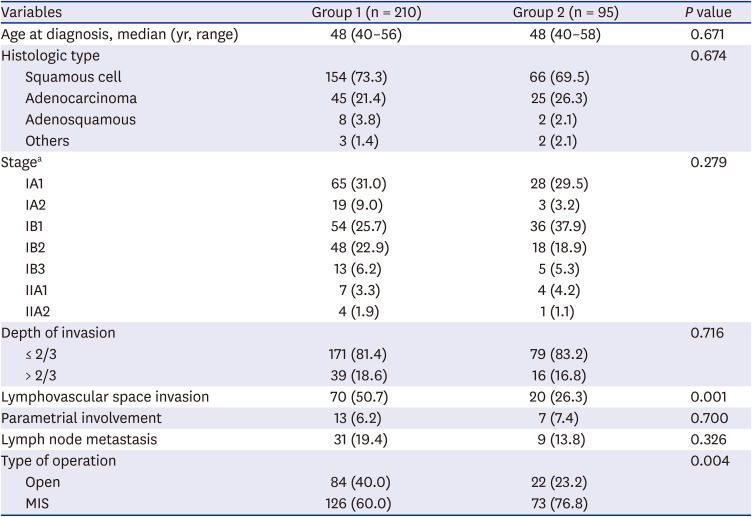
A total of 305 patients were included in this retrospective study, with 210 patients in group 1, and 95 patients in group 2. Their baseline characteristics are summarized in
Table 1. The two groups from different periods were relatively balanced in terms of their patients' demographics and tumor characteristics, except lymphovascular space invasion (
Table 1). Majority of cases in both groups were FIGO stage IB squamous cell carcinoma, accounting for 54.8% and 62.1% of cases in group 1 and group 2, respectively.
Table 1
Clinico-pathologic characteristics of the cervical cancer patients (n = 305)
|
Variables |
Group 1 (n = 210) |
Group 2 (n = 95) |
P value |
|
Age at diagnosis, median (yr, range) |
48 (40–56) |
48 (40–58) |
0.671 |
|
Histologic type |
|
|
0.674 |
|
Squamous cell |
154 (73.3) |
66 (69.5) |
|
Adenocarcinoma |
45 (21.4) |
25 (26.3) |
|
Adenosquamous |
8 (3.8) |
2 (2.1) |
|
Others |
3 (1.4) |
2 (2.1) |
|
Stagea
|
|
|
0.279 |
|
IA1 |
65 (31.0) |
28 (29.5) |
|
IA2 |
19 (9.0) |
3 (3.2) |
|
IB1 |
54 (25.7) |
36 (37.9) |
|
IB2 |
48 (22.9) |
18 (18.9) |
|
IB3 |
13 (6.2) |
5 (5.3) |
|
IIA1 |
7 (3.3) |
4 (4.2) |
|
IIA2 |
4 (1.9) |
1 (1.1) |
|
Depth of invasion |
|
|
0.716 |
|
≤ 2/3 |
171 (81.4) |
79 (83.2) |
|
> 2/3 |
39 (18.6) |
16 (16.8) |
|
Lymphovascular space invasion |
70 (50.7) |
20 (26.3) |
0.001 |
|
Parametrial involvement |
13 (6.2) |
7 (7.4) |
0.700 |
|
Lymph node metastasis |
31 (19.4) |
9 (13.8) |
0.326 |
|
Type of operation |
|
|
0.004 |
|
Open |
84 (40.0) |
22 (23.2) |
|
MIS |
126 (60.0) |
73 (76.8) |

Overall, minimally invasive surgery (MIS) was more frequently performed in group 2 than group 1 (60.0% vs. 76.8%,
P = 0.004), especially in earlier stage (stage IA, 72.6% vs. 100.0%,
P < 0.001; stage IB 52.2% VS. 69.5%,
P = 0.028). When performing subgroup analysis in stage IB cervical cancer, cases with tumor size smaller than 2 cm were treated by MIS in higher percentage in group 2 than group 1 (61.1% vs. 83.3%,
P = 0.024), while in larger tumor size (≥ 2 cm in diameter), ratio of open surgery and MIS did not show significant difference. Also, the median tumor diameter in stage IB cervical cancer cases treated by MIS was significantly smaller in group 2 (20 mm vs. 17 mm,
P = 0.015), while overall stage IB (25 mm vs. 18 mm,
P = 0.154) and cases treated by open method (26 mm vs. 34 mm,
P = 0.149) did not show significant difference. Rate of positive lymph node metastasis, in other words, the upstage rate was also significantly decreased in group 2 compared to group 1 in stage IB treated by MIS (28.0% vs. 7.7%,
P = 0.011) (
Table 2).
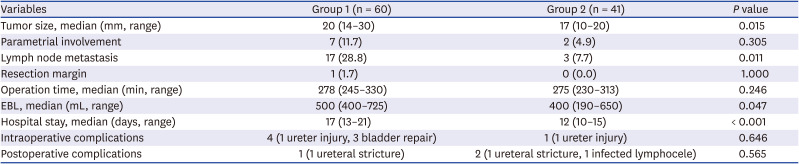
Table 2
Pathologic findings and operative details according to the study period in stage IB cervical cancer treated by minimally invasive surgery
|
Variables |
Group 1 (n = 60) |
Group 2 (n = 41) |
P value |
|
Tumor size, median (mm, range) |
20 (14–30) |
17 (10–20) |
0.015 |
|
Parametrial involvement |
7 (11.7) |
2 (4.9) |
0.305 |
|
Lymph node metastasis |
17 (28.8) |
3 (7.7) |
0.011 |
|
Resection margin |
1 (1.7) |
0 (0.0) |
1.000 |
|
Operation time, median (min, range) |
278 (245–330) |
275 (230–313) |
0.246 |
|
EBL, median (mL, range) |
500 (400–725) |
400 (190–650) |
0.047 |
|
Hospital stay, median (days, range) |
17 (13–21) |
12 (10–15) |
< 0.001 |
|
Intraoperative complications |
4 (1 ureter injury, 3 bladder repair) |
1 (1 ureter injury) |
0.646 |
|
Postoperative complications |
1 (1 ureteral stricture) |
2 (1 ureteral stricture, 1 infected lymphocele) |
0.565 |

Operative details of MIS performed in stage IB, such as operating time, intraoperative and postoperative complication did not show significant statistical differences between two groups, but hospital stay (17 days vs. 12 days,
P < 0.001) and estimated blood loss (500 mL vs. 400 mL,
P = 0.047) were significantly decreased in group 2 (
Table 2).
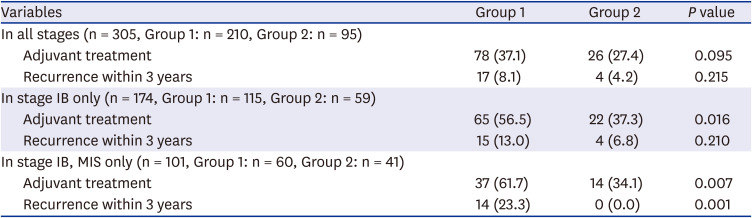
Overall, adjuvant treatment was less frequently performed in group 2 (37.1% vs. 27.4%,
P = 0.095), especially in stage IB (56.5% vs. 37.3%,
P = 0.016) (
Table 3). Despite decreased frequency in adjuvant treatment rate, there was no statistically significant difference in 3-year recurrence (8.1% in group 1 vs. 4.2% in group 2,
P = 0.215). Within stage IB, 3-year recurrence was significantly reduced in MIS cases (23.3% vs. 0%), while it was not statistically significant in total surgical cases (13.0% vs. 6.8%,
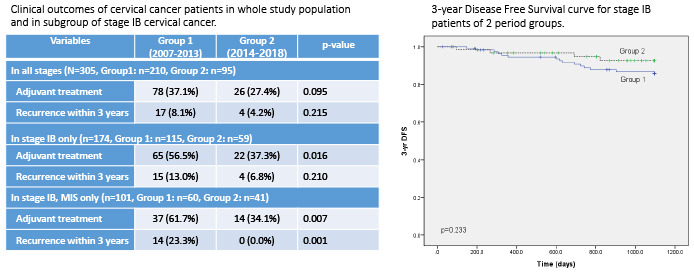
P = 0.210). 3-year disease free survivals (DFS) in stage IB was 87.0% and 93.2% in group 1 and 2, respectively, with no statistical difference (
P = 0.233), although the graph showed trend of longer 3-year DFS during period 2 (
Fig. 1). Due to short follow-up period and small number of death events, 3-year overall survival could not be analyzed.
Fig. 1
3-year disease free survival curve for stage IB patients of 2 period groups.
DFS = disease free survivals.
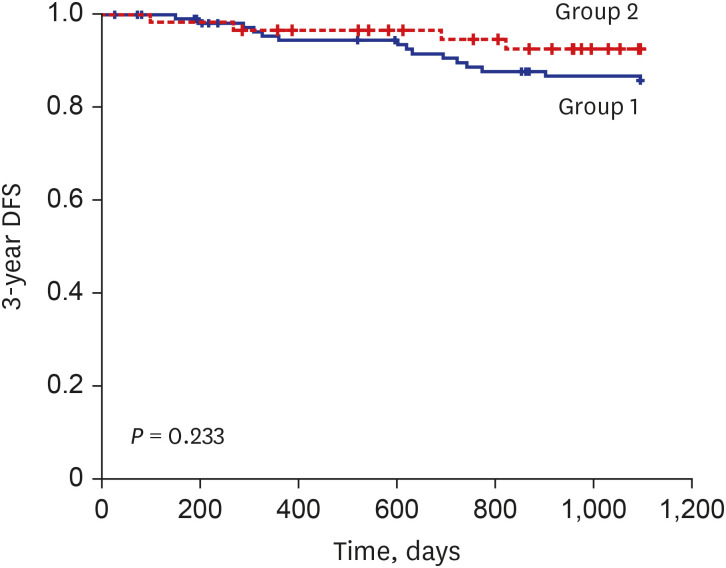

Table 3
Clinical outcomes of cervical cancer patients according to the study period in the whole study population and in the subgroup of stage IB cervical cancer
|
Variables |
Group 1 |
Group 2 |
P value |
|
In all stages (n = 305, Group 1: n = 210, Group 2: n = 95) |
|
|
|
|
Adjuvant treatment |
78 (37.1) |
26 (27.4) |
0.095 |
|
Recurrence within 3 years |
17 (8.1) |
4 (4.2) |
0.215 |
|
In stage IB only (n = 174, Group 1: n = 115, Group 2: n = 59) |
|
|
|
|
Adjuvant treatment |
65 (56.5) |
22 (37.3) |
0.016 |
|
Recurrence within 3 years |
15 (13.0) |
4 (6.8) |
0.210 |
|
In stage IB, MIS only (n = 101, Group 1: n = 60, Group 2: n = 41) |
|
|
|
|
Adjuvant treatment |
37 (61.7) |
14 (34.1) |
0.007 |
|
Recurrence within 3 years |
14 (23.3) |
0 (0.0) |
0.001 |

DISCUSSION
The present study evaluated effect of institutional, systemic QC on early cervical cancer management in terms of various quality indicators. We demonstrated that the tumor size treated with MIS in stage IB was significantly smaller in group 2, indicating stricter patient selection criteria was applied in result of QC. The percentage of adjuvant treatment performed in stage IB also decreased from 56.5% to 37.3% after implementation of QC. In addition, clinical outcomes of patients with stage IB treated by MIS were improved after QC program, partly due to better patient selection (3-year recurrence, 23.3% vs. 0%, P = 0.001).
In December 2013, our institution analyzed quality of care in our department, and realized patients' clinical outcomes were affected by surgeon factor, operation method, as well as perioperative care. Retrospective analysis of cervical cancer cases from 2007 to 2013 revealed that cervical cancer stage IB patients treated with MIS showed unusually shorter disease-free survival compared to patients treated with open surgery (3-year DFS 76.7% in MIS vs. 98.2% in open surgery,
P < 0.001), despite no difference in clinicopathologic characteristics between two groups (data unpublished). Also, unusually high adjuvant treatment rate of 56.5% was seen among stage IB patients during this time period, and unfortunately, a few cases that violated adjuvant treatment indications were found. In addition, high adjuvant treatment rates suggested candidates for radical surgery might also have been poorly chosen. Faced with such unfavorable results associated with early cervical cancer patient management, especially with MIS, we realized institutional intervention is imperative. Thus, we implemented monthly QC sessions in 2014 for proactive monitoring of surgeons' performance. Before such QC meetings started, operator decided specific treatment plans and surgical methods on his or her discretion. By providing constant feedback about such plans at departmental level, surgeons were enforced to adhere to guidelines and minimize personal differences while making clinical decisions. Although this was before the publication of LACC trial and other studies that suggested MIS might be more suitable for smaller tumor sizes,
121314 we came to consensus during the QC sessions that smaller tumor size (mostly tumor diameter < 2 cm) might be more suitable for MIS, to minimize manipulation and seeding of the tumor mass. We decided to apply stricter patient selection criteria and encouraged surgeons to perform MIS on smaller tumors, and reserve open radical hysterectomy for bigger, bulkier tumors. Also, adjuvant treatment indications for each patient were reviewed.
Various quality improvement systems to optimize clinical outcome and patient safety have been adapted to health care system, and measures of “quality” can be quantified by using different indicators.
345 Traditionally, quality of care has been evaluated from 3 different levels: structure, process, and outcome.
26 Structural indicators of quality include environmental features of care, such as trained personnel, hospital facilities, and equipment. Process indicators are performance measures in delivery of patient care, which include appropriate pre- and perioperative management, and adequate surgical process. Outcome indicators usually address the results of the treatments from the patient aspect, and most commonly used variables are surgical morbidity and mortality rates, recurrence and survival.
678 Since surgery is a highly technical procedure prone to individual variability in performance, many efforts have been made to standardize the procedure. Methods such as checklists or guidelines, accumulation of patient data, analysis of surgical outcome and detailed feedback on operative results to the surgeons have been tested in several institutions around the world.
2151617 Also in Korea, many efforts have been made to improve patient safety and quality of care in various fields of medicine, such as in regional trauma center as well as in NICU.
1819 To our knowledge, our study is the first to analyze the effect of institutional systemic QC on various quality indicators in early cervical cancer management.
For procedural indicators of QC, we analyzed patient selection criteria in terms of tumor size and operative details. After 2014, stage IA and majority of small tumor sized (< 2 cm) stage IB cervical cancer patients more frequently underwent MIS, which was demonstrated by smaller median tumor size in MIS compared to open method during period 2. This indicates stricter patient selection criteria for MIS was applied and inter-personal difference in management plans has been reduced. There have been several studies reporting open and direct feedback to surgeons regarding patient outcome and positive resection margins have generated discussions among surgeons and have led to reduction of heterogeneity within the group.
15 This type of internal QC has consistently been associated with improved clinical outcomes.
21517
For outcome indicators, we evaluated clinical outcome of patients by analyzing recurrence and survival. Overall 3-year recurrence and 3-year DFS did not show significant difference between 2 groups. When subgroup analysis of stage IB was performed, 3-year recurrence of MIS cases showed significant decrease to 0.0% in period 2 from 23.3% of period 1. Positive lymph node metastasis rate was also significantly decreased in MIS cases, suggesting decreased upstaging rate as well as adjuvant treatment rate after QC. Overall, inappropriately performed adjuvant treatment cases also declined due to QC. Improvements in some of these outcome indicators suggest that our monthly quality assurance programs encouraged surgeons to make better pre- and post-treatment decisions, and in turn, positively affected the clinical outcome of the cervical cancer patients.
Several limitations exist in our study. We compared two groups from two different time periods, and overtime, advancement in MIS technology, imaging modality and surgeon proficiency have taken place, which could have also contributed to better preoperative diagnosis as well as improved clinical outcomes of patients in period 2. Significantly smaller tumor size and decreased upstage rate in group 2 compared to group 1, although both results of better patient selection for surgery due to QC, could have also positively affected survival outcomes independently of QC. Another limitation is the retrospective and observational nature of the study with 12.7% of patients lost during 3-year follow-up period, with their oncologic outcomes unknown. In addition, we could not adjust all the factors, other than QC, that might have affected our outcome. Also, due to short follow-up period of group 2, we had to restrict our evaluation of recurrence to 3 years. But these are only general limitations of observational study. Our study is the first study to present single-institution experience of department-wide QC program implementation in efforts to oversee surgical management of early cervical cancer.
With recent controversy and suspicion surrounding MIS for early cervical cancer treatment, systemic monitoring should be considered for patient safety. Data need to be gathered and made into a database for regular analysis of the various quality indicators and discuss outcomes. Such efforts for QC need to be made into a national, furthermore, into an international effort. These continuous efforts toward quality care will eventually lead to improved clinical outcomes of cervical cancer patients.
ACKNOWLEDGMENTS
Authors thank Hye Ah Lee of Clinical Trial Center in Ewha Womans University Mokdong Hospital for advice on statistical analysis.
References
1. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019; 17(1):64–84. PMID:
30659131.

2. Verleye L, Vergote I, Reed N, Ottevanger PB. Quality assurance for radical hysterectomy for cervical cancer: the view of the European Organization for Research and Treatment of Cancer--Gynecological Cancer Group (EORTC-GCG). Ann Oncol. 2009; 20(10):1631–1638. PMID:
19556323.

3. du Bois A, Rochon J, Pfisterer J, Hoskins WJ. Variations in institutional infrastructure, physician specialization and experience, and outcome in ovarian cancer: a systematic review. Gynecol Oncol. 2009; 112(2):422–436. PMID:
18990435.

4. Arora V, Somashekhar SP. Essential surgical skills for a gynecologic oncologist. Int J Gynaecol Obstet. 2018; 143(Suppl 2):118–130. PMID:
30306588.

5. Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007; 105(3):801–812. PMID:
17433422.

6. Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000; 18(11):2327–2340. PMID:
10829054.

7. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018; 379(20):1895–1904. PMID:
30380365.

8. Rao ST, Nusrath S, Iyer RR, Patnaik SC, Saksena AR, Vanzar P, et al. Interpretation and implications of LACC trial. Indian J Gynecol Oncolog. 2019; 17(2):39.

9. Köhler C, Schneider A, Marnitz S, Plaikner A. The basic principles of oncologic surgery during minimally invasive radical hysterectomy. J Gynecol Oncol. 2020; 31(1):e33. PMID:
31833260.

10. Vergote I, Magrina JF, Zanagnolo V, Magtibay PM, Butler K, Gil-Moreno A, et al. The LACC trial and minimally invasive surgery in cervical cancer. J Minim Invasive Gynecol. 2020; 27(2):462–463. PMID:
31520725.

11. Kimmig R, Ind T. Minimally invasive surgery for cervical cancer: consequences for treatment after LACC Study. J Gynecol Oncol. 2018; 29(4):e75. PMID:
29770634.

12. Li P, Chen L, Ni Y, Liu J, Li D, Guo J, et al. Comparison between laparoscopic and abdominal radical hysterectomy for stage IB1 and tumor size <2 cm cervical cancer with visible or invisible tumors: a multicentre retrospective study. J Gynecol Oncol. 2021; 32(2):e17. PMID:
33470062.

13. Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. 2019; 153(1):3–12. PMID:
30642625.

14. Chen C, Liu P, Ni Y, Tang L, Xu Y, Bin X, et al. Laparoscopic versus abdominal radical hysterectomy for stage IB1 cervical cancer patients with tumor size ≤ 2 cm: a case-matched control study. Int J Clin Oncol. 2020; 25(5):937–947. PMID:
32062731.
15. Stranne J, Axen E, Franck-Lissbrant I, Fransson P, Frånlund M, Hugosson J, et al. Single institution followed by national implementation of systematic surgical quality control and feedback for radical prostatectomy: a 20-year journey. World J Urol. 2020; 38(6):1397–1411. PMID:
31388817.

16. Biau DJ, Resche-Rigon M, Godiris-Petit G, Nizard RS, Porcher R. Quality control of surgical and interventional procedures: a review of the CUSUM. Qual Saf Health Care. 2007; 16(3):203–207. PMID:
17545347.

17. Murzi M, Cerillo AG, Bevilacqua S, Gasbarri T, Kallushi E, Farneti P, et al. Enhancing departmental quality control in minimally invasive mitral valve surgery: a single-institution experience. Eur J Cardiothorac Surg. 2012; 42(3):500–506. PMID:
22427391.

18. Huh Y, Kwon J, Moon J, Kang BH, Kim S, Yoo J, et al. An evaluation of the effect of performance improvement and patient safety program implemented in a new regional trauma center of Korea. J Korean Med Sci. 2021; 36(22):e149. PMID:
34100561.

19. Chang YS. Moving forward to improve safety and quality of neonatal intensive care in Korea. J Korean Med Sci. 2018; 33(9):e89. PMID:
29441743.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download