This article has been
cited by other articles in ScienceCentral.
Abstract
As the number of people vaccinated increases, people who complain of adverse reactions continue to occur. We experienced a case characterized by low blood pressure, persistent fever, edema due to increased systemic vascular permeability, and systemic inflammation confirmed by image and laboratory examinations after ChAdOx1 coronavirus disease 2019 (COVID-19) vaccination. The diagnostic criteria for multisystem inflammatory syndrome (MIS) in adults are known as fever of 3 days or more in adults, 2 or more mucocutaneous/gastrointestinal/neurologic symptoms, elevation of inflammatory markers, and clinical/imaging diagnosis of heart failure. A 67-year-old man who was medicated for hypertension and diabetes was admitted complaining of fever, maculopapular rash, diarrhea, headache, chills, and dizziness 6 days after the first vaccination of ChAdOx1 nCoV-19 in Korea. The COVID-19 test was negative but with low blood pressure, leukocytosis, skin rash, pulmonary edema, and increased inflammation markers. His lab findings and clinical course were consistent with those of MIS after COVID-19 vaccination. He was medicated with methylprednisolone 1 mg/kg and diuretics and recovered rapidly. He was discharged after 2 weeks and confirmed cure at outpatient clinic. We report an MIS case after COVID-19 vaccination in Korea.
Go to :

Graphical Abstract
Go to :

Keywords: Coronavirus Disease 2019 (COVID-19), COVID-19 Vaccination, Multisystem Inflammatory Syndrome, Methylprednisolone
INTRODUCTION
We are living in the coronavirus disease 2019 (COVID-19) pandemic era and are actively introducing vaccination as a way to overcome it. As of July 12, 2021, the Republic of Korea was counted as having completed 30.4% of the primary vaccination. As the number of people vaccinated increases, people who complain of adverse reactions continue to occur.
1 The case definition of multisystem inflammatory syndrome in adults 21 years of age or older includes the following: 1) severe dysfunction of one or more extra-pulmonary organs in association with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), 2) evidence of severe inflammation.
2 Cases of multisystem inflammatory syndrome have been reported after COVID-19 vaccination,
34 and we have experienced similar cases. Therefore, we would like to describe this case.
Go to :

CASE DESCRIPTION
A 67-year-old male patient was taking medication for hypertension and diabetes. He received his first dose of ChAdOx1 nCoV-19 vaccine on June 1, 2021, and since June 7, symptoms of fever, maculopapular rash, diarrhea, headache, chills, and dizziness continued, and he was hospitalized at a local hospital. The COVID-19 polymerase chain reaction (PCR) performed at the local hospital on June 13 was negative. However, the fever could not be controlled continuously, so on June 14, he was transferred through the emergency room of the hospital. He had no prior diagnosis of COVID-19 and had no close contact with known COVID-19 patients. At the time of admission, his vital signs were blood pressure 90/60 mmHg, pulse rate 106/min, respiratory rate 20/min, body temperature 38.9°C, and O2 saturation was 95%. At the time the patient was admitted, the result of the COVID-19 PCR test was negative. His lab findings were as follows: white blood cell (WBC) 18,790 (neutrophil 88.9%), hemoglobin 13.6 g/dL, platelet count 158,000/μL, BUN/Cr 17.0/1.07 mg/dL (eGFR 71.45), aspartate transaminase (AST)/alanine aminotransferase (ALT) 15/87 IU/L, lactate dehydrogenase (LDH) 228 U/L, ferritin 1,948.0 ng/mL, lactic acid 32.0 mg/dL, C-reactive protein (CRP) 247.28 mg/L, procalcitonin 4.420 ng/mL, adenosis deaminase (ADA) 33.7 IU/L, and B-type natriuretic peptide (BNP) 4,687.0 pg/mg. The anti-nuclear antibody was 1:40 (weak positive), the anti-double-stranded DNA antibody was negative, and the rheumatoid factor was 12.1 IU/mL (normal range, 0–15). The level of triglycerides was 183 mg/dL (normal range, 0–200). Blood culture, urine and sputum results were all confirmed as negative. Arterial blood gas analysis (aBGA) result was 7.404-34.0-70.8-20.8-94.8%. Proteinuria was confirmed in a 24-hour urine chemistry test (total protein 344.586 mg/day). Natural killer (NK) cell activity was also checked, and it was confirmed that the activity was very low at 1 pg/mL. Chest computed tomography (CT) performed at an external hospital showed no specific lesions other than a 1 cm sized anterior mediastinal nodule. No specific lesions other than fatty liver were observed in abdomen pelvic CT (APCT). Echocardiography was performed to differentiate heart failure due to BNP elevation. The echocardiography findings were confirmed by normal left ventricle contractility without definite regional wall motion abnormality, trivial tricuspid valve regurgitation with normal pulmonary arterial pressure, and moderate left atrial enlargement. However, as his hospitalization length increased, pulmonary edema gradually increased and he gained weight (at the time of admission he weighed 86.6 kg and after 12 days he reached his maximum weight of 96.8 kg).
Because the fever persisted, inflammatory markers were elevated, and relative bradycardia was present, it was difficult to rule out the possibility of salmonellosis, so we started ciprofloxacin. We performed Positron Emission Tomography-CT (PET-CT) to determine the cause of inflammation. PET-CT showed reactive lymph nodes (Lt. cervical chain, Lt. supraclavicular regions, both axillae, mediastinum, portocaval, pericaval, Lt. paraaortic, bilateral external iliac chains, inguinal regions), and splenic hyperplasia. No other focal inflammatory lesions were observed (
Fig. 1).
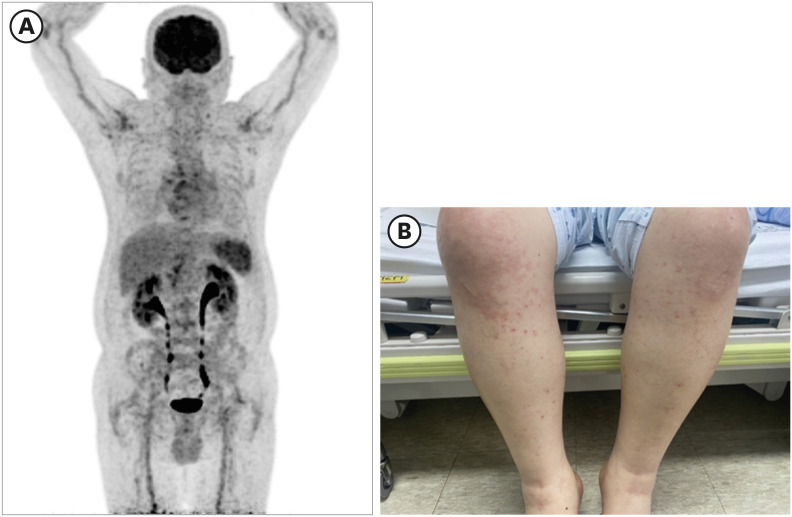 | Fig. 1
Clinical features of the patient. (A) Patient's Positron Emission Tomography findings: Lt. cervical chain, Lt. There are nodular FDG uptakes in the supraclavicular region, both axillae and mediastinum. Moreover, there is nodular uptake in the portocaval (SUVmax: 4.06) and pericaval (3.54) regions. Lt. Mild nodular uptakes in the paraaortic region, bilateral external iliac chains, and inguinal regions. Spleen's FDG uptake increased overall. (B) Maculopapular rash finding developed in the patient.
FDG = fluorodeoxyglucose.

|
The diagnostic criteria for MIS-A are known as fever of 3 days or more in adults, 2 or more mucocutaneous/gastrointestinal/neurological symptoms, elevation of inflammatory markers, and clinical/imaging diagnosis of heart failure. The patient had a fever, diarrhea, rash and neurological symptoms such as dizziness that had persisted for more than 3 days. The patient's examination results showed a decrease in blood pressure, an increase in the markers of inflammation (C-reactive protein/ferritin/procalcitonin), neutrophilia, and pulmonary edema. Since the patient met all five of these criteria, we were able to diagnose this patient's symptoms using the MIS-A result (2). So, we started using methylprednisolone 1 mg/kg and a diuretic (Furosemide). One day after steroid use, fever was normalized from 38.7°C to 36.5°C (
Fig. 2), O
2 saturation was maintained well over 97% in room air, and blood pressure was also maintained stably. The procalcitonin level was also improved to 0.460 ng/mL. After 2 weeks of steroid application, the patient was discharged, and just before discharge, the body weight improved to a normal level (81.6 kg), and the labs also recovered (CBC WBC count 12,730 (neutrophil 51.3%) BUN/Cr 20.0/0.75 mg/dL (eGFR 94.92), CRP 3.34 mg/L, lactic acid 14.0 mg/dL). Ferritin was still confirmed at 1,051 ng/mL when followed up at the outpatient clinic 2 weeks after discharge (
Fig. 2).
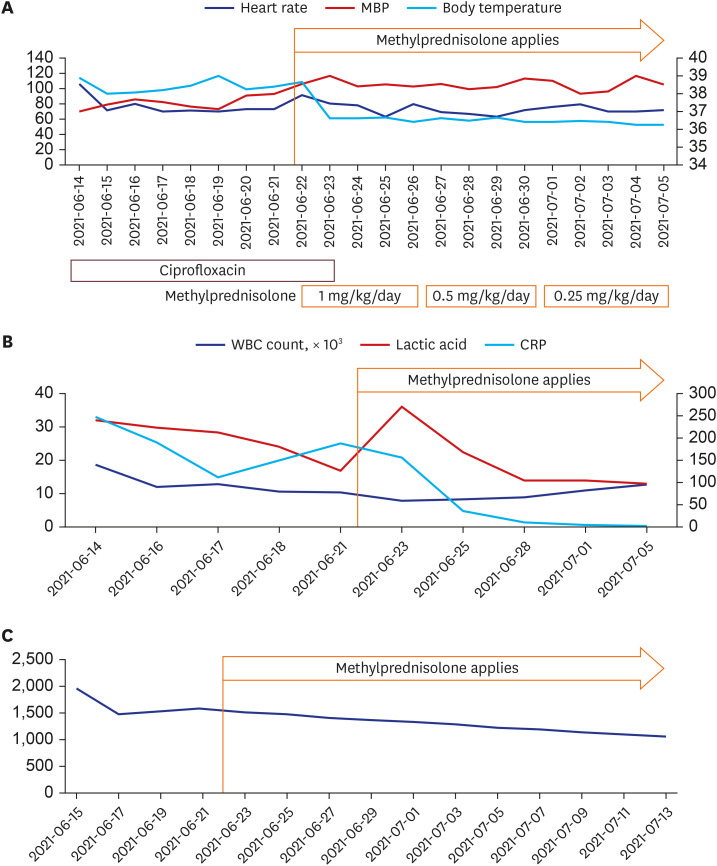 | Fig. 2
Patient's clinical course: changes in vital sign indicators and laboratory results. (A) Vital sings; (B) Laboratory tests: WBC count, lactic acid, CRP (C) Ferritin levels (ng/mL).
MBP = mean blood pressure, WBC = white blood cell, CRP = C-reactive protein.

|
Go to :

DISCUSSION
We experienced a case characterized by low blood pressure, persistent fever, edema due to increased systemic vascular permeability, and systemic inflammation confirmed by image and lab examinations after COVID-19 vaccination.
Cases of systemic vasculitis have been reported not only with the COVID-19 vaccine, but also with several other vaccinations. In particular, since vaccination is mainly conducted on children, a systematic review has reported that Kawasaki's disease can occur after vaccination.
5 Kawasaki's disease is a disease characterized by systemic inflammation, particularly vasculitis, and can be thought of as a disease similar to the multisystem inflammatory syndrome presumed in our case.
Multisystem inflammatory syndrome (MIS) was a concept initially introduced in children (MIS-C). MIS-C is a febrile hyper-inflammatory syndrome developed in children with recent SARS-CoV-2 infection and is known to cause symptoms similar to Kawasaki's disease.
6 Although rare, similar symptoms occurred in adults, so the concept of MIS in adult (MIS-A) was introduced.
78 The case definition of MIS-A is as follows: 1) In adults 21 years of age or older; 2) If fever persists for more than 3 days; 3) Two or more mucocutaneous symptoms, gastrointestinal symptoms, hypotension, and neurologic symptoms; 4) Elevation of inflammatory markers (ESR, CRP, ferritin, or procalcitonin); 5) Two or more of BNP elevation, neutrophilia/thrombocytopenia, clinical or echocardiography evidence of heart failure, or myo-pericarditis in EKG.
2
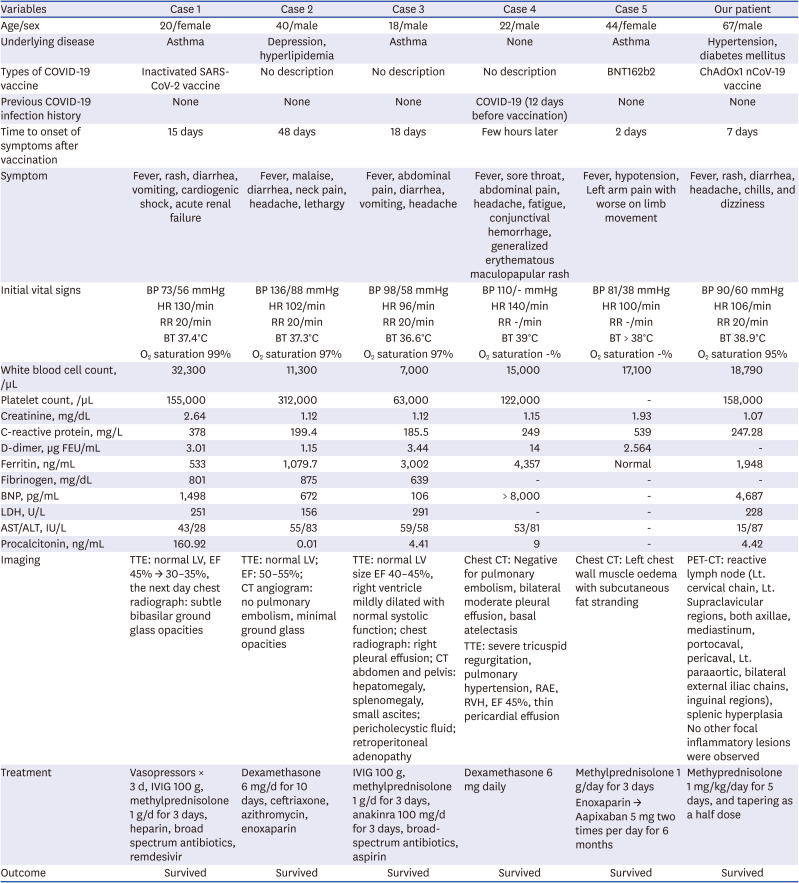
MIS-A cases after COVID-19 vaccination are rare. When searching Pubmed with “COVID-19 vaccine” AND “multisystem inflammatory syndrome,” 5 cases have been reported. These 5 cases and our case are summarized and described in
Table 1.
349 Most cases were vaccinated with the COVID-19 vaccine within 2 to 6 weeks of onset of symptoms. There were different types of COVID-19 vaccines. They complained of various systemic illness symptoms (fever, rash, sore throat, dyspnea on exertion, headache, abdominal pain with diarrhea, etc.). Their lab data also showed variation. There were cases in which leukocytosis was severe (32,300/μL), and there was a case in which WBC count was normal (7,000/μL) but thrombocytopenia (63,000/μL) occurred. The ferritin level also varied from 533 to 3,002 ng/mL, and the degree of elevation of BNP also varied from 106 to 1,498 pg/mL. In common, CRP level and procalcitonin level showed a tendency to rise, and LDH was almost normal level. Their symptoms improved after using methylprednisolone and IVIG.
4 One special case occurred in Abu Dhabi, United Arab Emirates, this patient was previously confirmed to have COVID-19 and was vaccinated 2 times, 4 weeks apart, and the symptoms developed a few hours later.
3
Table 1
Cases of multisystemic inflammatory syndrome in adults after COVID-19 vaccination: review of literature
|
Variables |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
Our patient |
|
Age/sex |
20/female |
40/male |
18/male |
22/male |
44/female |
67/male |
|
Underlying disease |
Asthma |
Depression, hyperlipidemia |
Asthma |
None |
Asthma |
Hypertension, diabetes mellitus |
|
Types of COVID-19 vaccine |
Inactivated SARS-CoV-2 vaccine |
No description |
No description |
No description |
BNT162b2 |
ChAdOx1 nCoV-19 vaccine |
|
Previous COVID-19 infection history |
None |
None |
None |
COVID-19 (12 days before vaccination) |
None |
None |
|
Time to onset of symptoms after vaccination |
15 days |
48 days |
18 days |
Few hours later |
2 days |
7 days |
|
Symptom |
Fever, rash, diarrhea, vomiting, cardiogenic shock, acute renal failure |
Fever, malaise, diarrhea, neck pain, headache, lethargy |
Fever, abdominal pain, diarrhea, vomiting, headache |
Fever, sore throat, abdominal pain, headache, fatigue, conjunctival hemorrhage, generalized erythematous maculopapular rash |
Fever, hypotension, Left arm pain with worse on limb movement |
Fever, rash, diarrhea, headache, chills, and dizziness |
|
Initial vital signs |
BP 73/56 mmHg |
BP 136/88 mmHg |
BP 98/58 mmHg |
BP 110/- mmHg |
BP 81/38 mmHg |
BP 90/60 mmHg |
|
HR 130/min |
HR 102/min |
HR 96/min |
HR 140/min |
HR 100/min |
HR 106/min |
|
RR 20/min |
RR 20/min |
RR 20/min |
RR -/min |
RR -/min |
RR 20/min |
|
BT 37.4°C |
BT 37.3°C |
BT 36.6°C |
BT 39°C |
BT > 38°C |
BT 38.9°C |
|
O2 saturation 99% |
O2 saturation 97% |
O2 saturation 97% |
O2 saturation -% |
O2 saturation -% |
O2 saturation 95% |
|
White blood cell count, /µL |
32,300 |
11,300 |
7,000 |
15,000 |
17,100 |
18,790 |
|
Platelet count, /µL |
155,000 |
312,000 |
63,000 |
122,000 |
- |
158,000 |
|
Creatinine, mg/dL |
2.64 |
1.12 |
1.12 |
1.15 |
1.93 |
1.07 |
|
C-reactive protein, mg/L |
378 |
199.4 |
185.5 |
249 |
539 |
247.28 |
|
D-dimer, µg FEU/mL |
3.01 |
1.15 |
3.44 |
14 |
2.564 |
- |
|
Ferritin, ng/mL |
533 |
1,079.7 |
3,002 |
4,357 |
Normal |
1,948 |
|
Fibrinogen, mg/dL |
801 |
875 |
639 |
- |
- |
- |
|
BNP, pg/mL |
1,498 |
672 |
106 |
> 8,000 |
- |
4,687 |
|
LDH, U/L |
251 |
156 |
291 |
- |
- |
228 |
|
AST/ALT, IU/L |
43/28 |
55/83 |
59/58 |
53/81 |
- |
15/87 |
|
Procalcitonin, ng/mL |
160.92 |
0.01 |
4.41 |
9 |
- |
4.42 |
|
Imaging |
TTE: normal LV, EF 45% → 30–35%, the next day chest radiograph: subtle bibasilar ground glass opacities |
TTE: normal LV; EF: 50–55%; CT angiogram: no pulmonary embolism, minimal ground glass opacities |
TTE: normal LV size EF 40–45%, right ventricle mildly dilated with normal systolic function; chest radiograph: right pleural effusion; CT abdomen and pelvis: hepatomegaly, splenomegaly, small ascites; pericholecystic fluid; retroperitoneal adenopathy |
Chest CT: Negative for pulmonary embolism, bilateral moderate pleural effusion, basal atelectasis |
Chest CT: Left chest wall muscle oedema with subcutaneous fat stranding |
PET-CT: reactive lymph node (Lt. cervical chain, Lt. Supraclavicular regions, both axillae, mediastinum, portocaval, pericaval, Lt. paraaortic, bilateral external iliac chains, inguinal regions), splenic hyperplasia No other focal inflammatory lesions were observed |
|
TTE: severe tricuspid regurgitation, pulmonary hypertension, RAE, RVH, EF 45%, thin pericardial effusion |
|
Treatment |
Vasopressors × 3 d, IVIG 100 g, methylprednisolone 1 g/d for 3 days, heparin, broad spectrum antibiotics, remdesivir |
Dexamethasone 6 mg/d for 10 days, ceftriaxone, azithromycin, enoxaparin |
IVIG 100 g, methylprednisolone 1 g/d for 3 days, anakinra 100 mg/d for 3 days, broad-spectrum antibiotics, aspirin |
Dexamethasone 6 mg daily |
Methylprednisolone 1 g/day for 3 days |
Methyprednisolone 1 mg/kg/day for 5 days, and tapering as a half dose |
|
Enoxaparin → Aapixaban 5 mg two times per day for 6 months |
|
Outcome |
Survived |
Survived |
Survived |
Survived |
Survived |
Survived |

In our case, persistent fever and hypotension occurred, and clinical symptoms of diarrhea and heart failure were accompanied by elevation of CRP, ferritin, and procalcitonin levels, so it met the MIS-A case definition. In addition to the symptoms of case definition, symptoms such as systemic edema and pleural effusion presumably caused by an increase in vascular permeability due to systemic inflammation were also accompanied. A characteristic feature of the lab results was a decrease in NK cell activity. Although it is difficult to know the exact relationship with the pathophysiology of MIS, it is presumed that it is probably related to the abnormal activation of innate immunity.
Several reports describe the possibility of exacerbation of an autoimmune disease after the application of the COVID-19 vaccine.
101112 This turned out to be due to the cascade of immune reaction after vaccination. In other words, the COVID-19 vaccine is recognized by MHC I and II molecules in antigen presenting cells and activates T cells and B cells. In antigen presenting cells, mRNA detects TLR7 and 8, resulting in the activation of the descending cascade and the secretion of pro-inflammatory cytokines and type I interferons is stimulated.
13 We speculate that this cascade of immune responses may have had a significant impact on the induction of MIS-A in our patients.
We checked the possibility of MIS-A caused by the COVID-19 vaccine using various tools such as PET-CT. Since there are not many reports of it, we think it will be helpful to physicians treating adverse reactions. So far, no cases of death have been reported as a result of MIS following vaccination. However, as the number of COVID-19 vaccinations increases, the incidence of related cases will also increase, and it is thought that more severe cases will occur among them. Therefore, in the future, it will be necessary to collect and accurately analyze the adverse reactions of vaccination, especially cases related to MIS.
Looking at cases of MIS-A, cases occurring after infection with SARS-CoV-2 are slightly more common and appear to progress to slightly more severe severity than cases occurring after vaccination.
8 Therefore, we do not believe that it is necessary not to vaccinate because of the adverse reactions. In addition, it is necessary for doctors to closely monitor and treat patients with systemic inflammation after vaccination.
COVID-19 vaccines are the most powerful weapon to end the pandemic, but concerns about rare and threatening adverse reactions are deterring some people from getting the vaccine. MIS is known to be a rare consequence of COVID-19 infection and there are few reports indicating that a similar clinical syndrome can occur with vaccination alone. Our case is significant insofar as it is the first to be reported in Korea, especially in Asia. Doctors should check for any rare and serious adverse reactions after vaccination to ensure safe vaccination.
Ethics statement
This report was approved by our Institutional Review Board, and the requirement for informed consent was waived (subject number: SCHCA 2021-10-009).
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download