Abstract
Notes
AUTHOR CONTRIBUTIONS
Conception or design: J.H.L., K.H.Y., S.H.K.
Acquisition, analysis, or interpretation of data: J.H.L., S.Y.L., S.A.C., C.J.H., A.R.J., K.R.K., S.H.K.
Drafting the work or revising: J.H.L., K.H.Y., S.H.K.
Final approval of the manuscript: J.H.L., S.Y.L., S.A.C., C.J.H., A.R.J., K.R.K., K.H.Y., S.H.K.
ACKNOWLEDGMENTS
REFERENCES
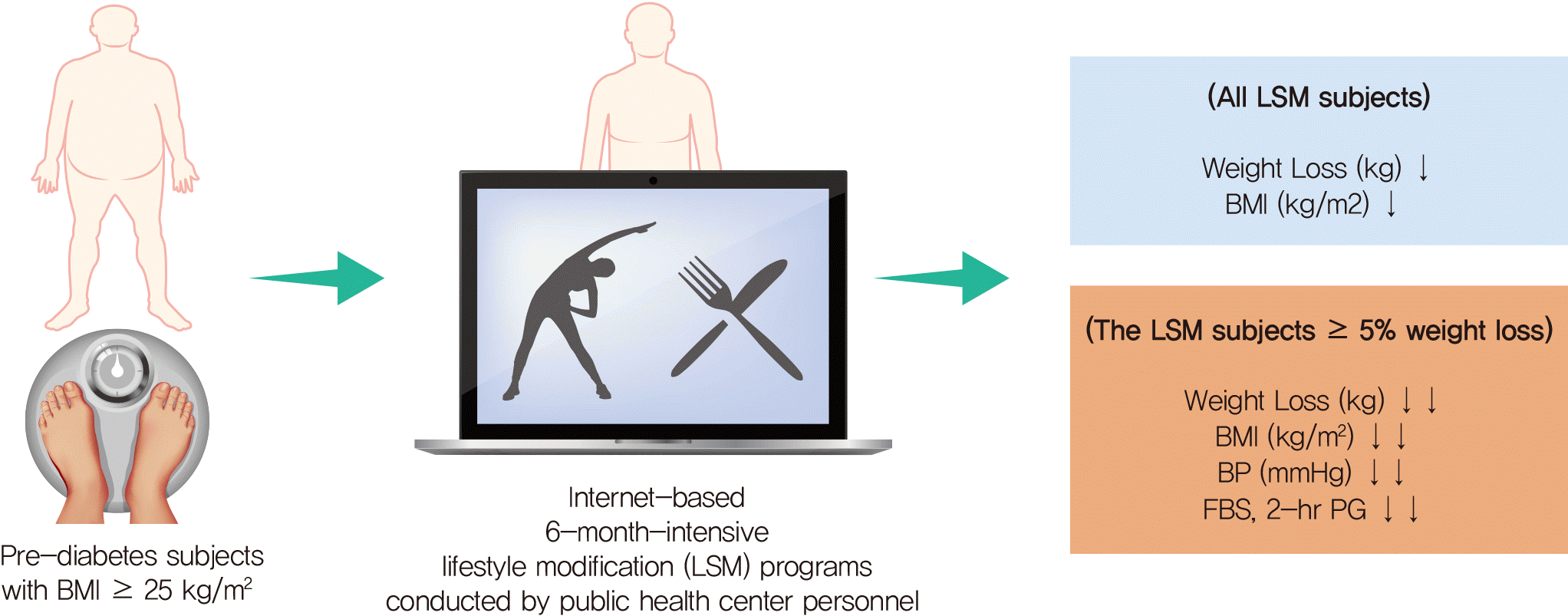
Fig. 1.
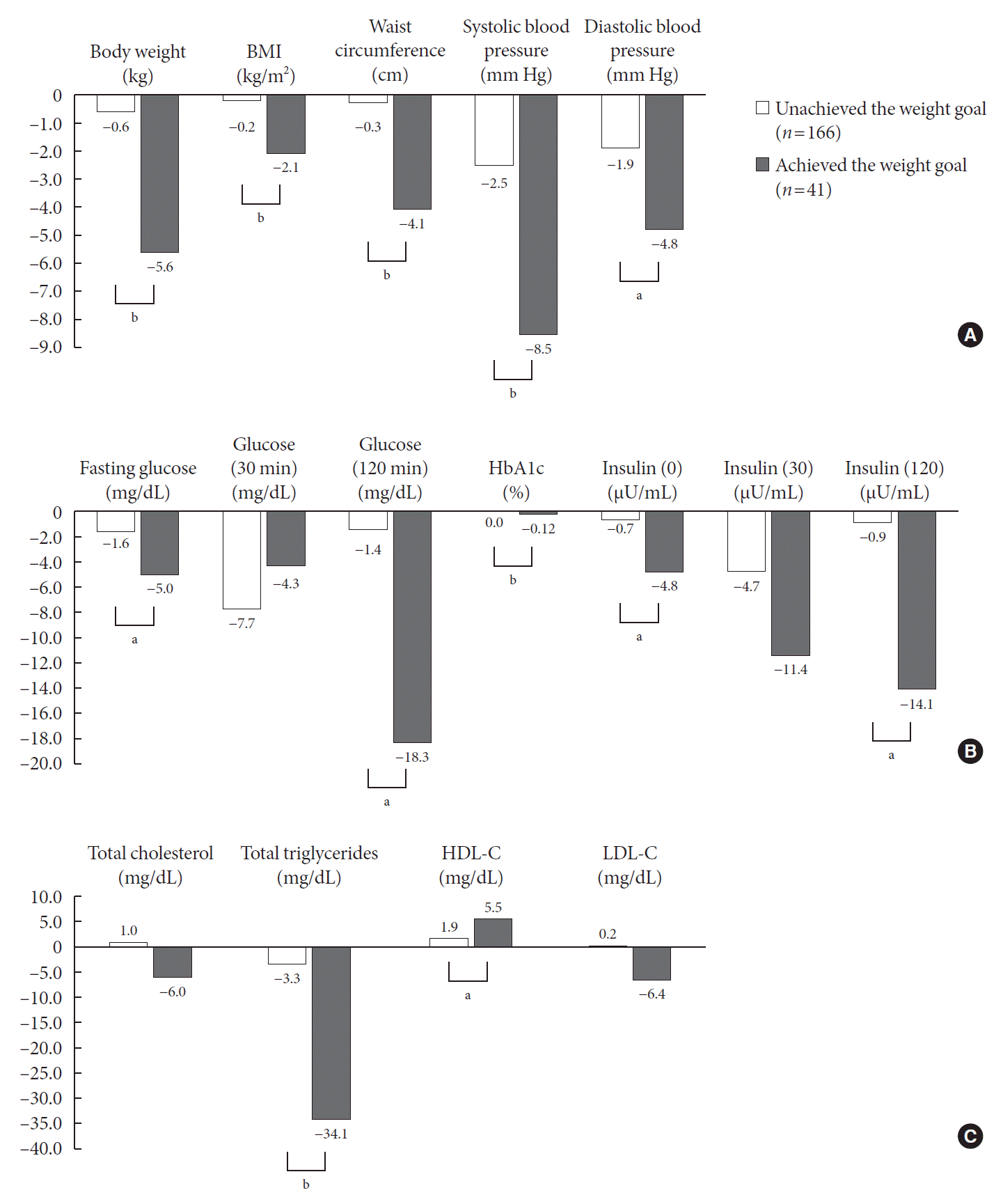
Table 1.
| Variable |
LSM (n=207) |
SM (n=208) |
P valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Baseline | 6 Months | Mean change | P valuea | No. | Baseline | 6 Months | Mean change | P valuea | ||
| Body weight, kg | 207 | 71.1±10.9 | 69.4±10.9 | –1.6 | <0.001 | 208 | 69.4±10.1 | 69.0±10.2 | –0.4 | 0.002 | <0.001 |
| BMI, kg/m2 | 207 | 26.5±3.0 | 26.0±3.0 | –0.6 | <0.001 | 208 | 26.0±2.6 | 25.9±2.7 | –0.1 | 0.001 | <0.001 |
| Waist circumference, cm | 206 | 91.4±8.1 | 90.4±8.0 | –1.1 | <0.001 | 208 | 90.7±7.2 | 90.6±7.3 | –0.1 | 0.670 | 0.003 |
| Blood pressure, mm Hg | |||||||||||
| Systolic | 206 | 122.0±11.9 | 118.4±12.6 | –3.7 | <0.001 | 205 | 122.6±11.2 | 120.7±10.7 | –2.0 | 0.001 | 0.051 |
| Diastolic | 206 | 81.9±8.5 | 79.5±9.0 | –2.5 | <0.001 | 205 | 81.9±8.1 | 80.1±8.2 | –1.8 | <0.001 | 0.256 |
| Fasting glucose, mg/dL | 207 | 100.1±8.7 | 97.8±9.9 | –2.3 | <0.001 | 208 | 98.6±8.9 | 97.8±9.6 | –0.9 | 0.077 | 0.102 |
| Glucose, 30 min, mg/dL | 206 | 174.7±24.0 | 167.7±25.6 | –7.0 | <0.001 | 207 | 171.4±25.3 | 169.7±28.5 | –1.7 | 0.305 | 0.021 |
| Glucose, 120 min, mg/dL | 207 | 135.3±31.2 | 130.5±34.2 | –4.8 | 0.026 | 208 | 135.9±29.8 | 135.9±36.1 | 0.0 | 0.990 | 0.140 |
| HbA1c, % | 205 | 5.78±0.30 | 5.76±0.30 | –0.02 | 0.059 | 206 | 5.76±0.30 | 5.77±0.34 | 0.01 | 0.580 | 0.100 |
| Insulin, 0, μU/mL | 191 | 8.8±8.7 | 7.2±4.4 | –1.6 | 0.002 | 194 | 8.1±5.3 | 8.2±4.7 | 0.0 | 0.742 | 0.011 |
| Insulin, 30, μU/mL | 191 | 43.2±36.3 | 37.1±33.6 | –6.1 | 0.001 | 193 | 42.7±26.2 | 43.3±29.5 | 0.6 | 0.937 | 0.015 |
| Insulin, 120, μU/mL | 192 | 43.2±28.5 | 39.6±29.7 | –3.6 | 0.011 | 194 | 46.8±32.8 | 47.9±38.1 | 1.0 | 0.767 | 0.079 |
| HOMA-IR | 191 | 2.2±2.1 | 1.8±1.2 | –0.4 | <0.001 | 194 | 2.0±1.4 | 2.0±1.2 | 0.0 | 0.951 | 0.007 |
| HOMA-β | 191 | 88.1±92.1 | 78.6±49.7 | –9.5 | 0.278 | 194 | 85.1±57.1 | 88.7±57.3 | 3.6 | 0.233 | 0.090 |
| Total cholesterol, mg/dL | 205 | 191.1±31.4 | 190.8±32.7 | –0.4 | 0.853 | 205 | 189.2±36.0 | 190.5±33.2 | 1.2 | 0.352 | 0.541 |
| Total triglycerides, mg/dL | 205 | 155.0±94.9 | 145.7±98.0 | –9.3 | 0.002 | 205 | 153.7±92.8 | 156.8±94.7 | 3.1 | 0.330 | 0.003 |
| HDL-C, mg/dL | 205 | 50.6±12.3 | 53.2±12.7 | 2.6 | <0.001 | 205 | 51.9±13.7 | 52.5±13.9 | 0.5 | 0.260 | 0.001 |
| LDL-C, mg/dL | 203 | 121.5±30.0 | 120.4±32.0 | –1.1 | 0.549 | 204 | 119.1±33.6 | 120.2±30.6 | 1.1 | 0.353 | 0.358 |
Values are presented as mean±standard deviation.
LSM, lifestyle modification; SM, standard management; BMI, body mass index; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-β, homeostatic model assessment for β-cell function; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download