1. nanditha a, ma rc, ramachandran a, snehalatha c, chan jc, chia ks, et al. diabetes in asia and the pacific: implications for the global epidemic. diabetes care. 2016; 39:472–85.

2. Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019; 43:487–94.

3. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007; 30:753–9.

4. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41:2669–701.

5. Cai X, Gao X, Yang W, Han X, Ji L. Efficacy and safety of initial combination therapy in treatment-naive type 2 diabetes patients: a systematic review and meta-analysis. Diabetes Ther. 2018; 9:1995–2014.
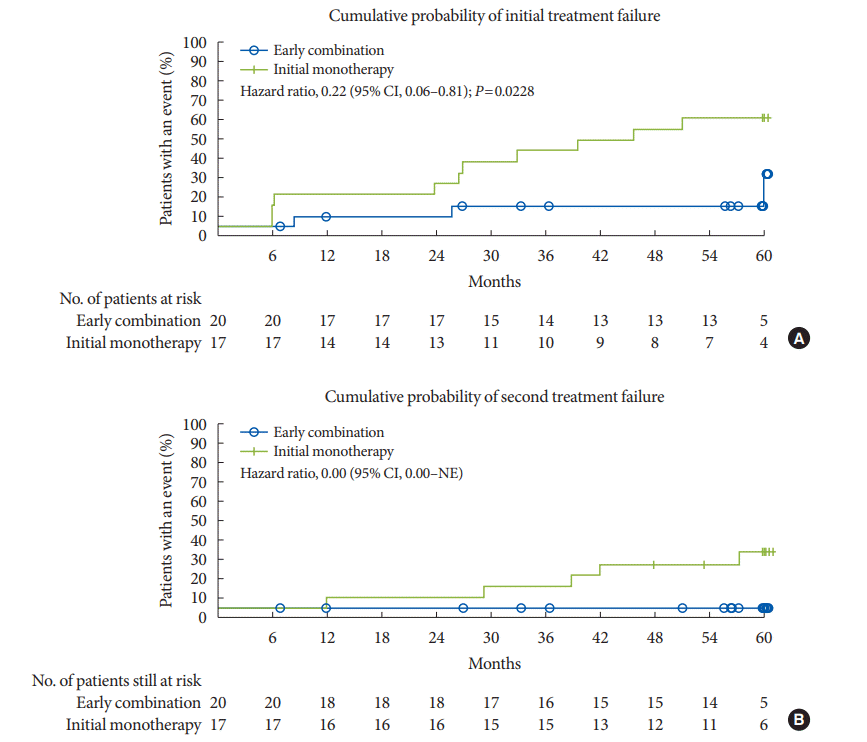
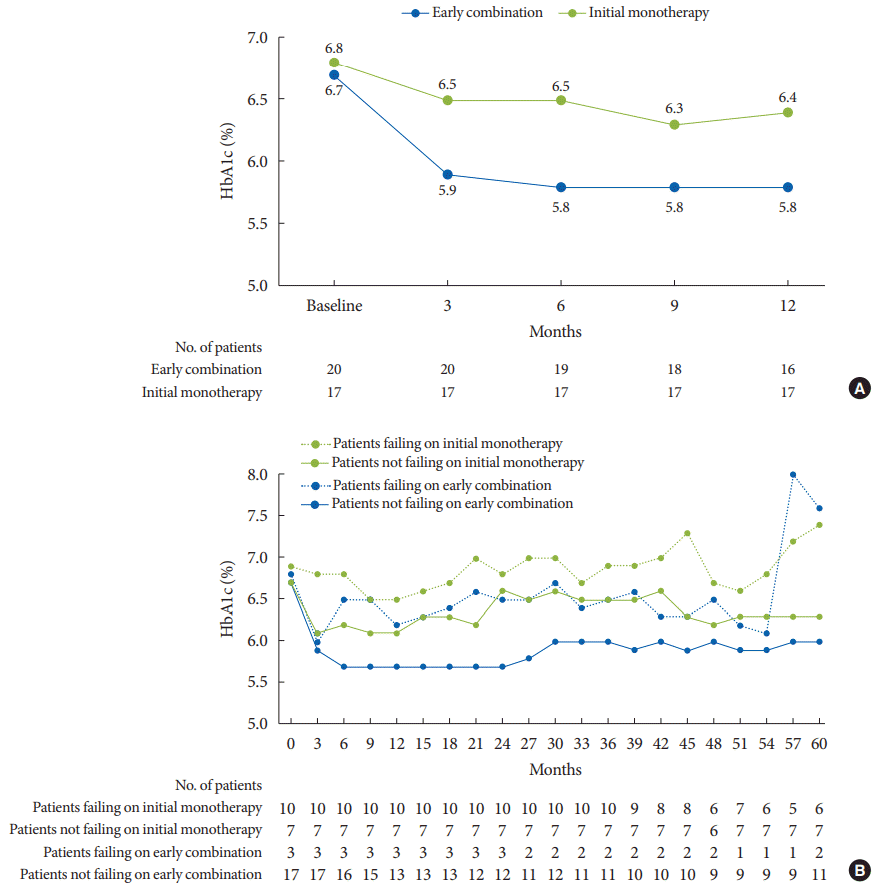
6. Matthews DR, Paldanius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019; 394:1519–29.

7. Del Prato S, Foley JE, Kothny W, Kozlovski P, Stumvoll M, Paldanius PM, et al. Study to determine the durability of glycaemic control with early treatment with a vildagliptin-metformin combination regimen vs. standard-of-care metformin monotherapy-the VERIFY trial: a randomized double-blind trial. Diabet Med. 2014; 31:1178–84.
8. Matthews DR, Paldanius PM, Stumvoll M, Han J, Bader G, Chiang Y, et al. A pre-specified statistical analysis plan for the VERIFY study: vildagliptin efficacy in combination with metformin for early treatment of T2DM. Diabetes Obes Metab. 2019; 21:2240–7.

9. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:487–93.

10. Ahren B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009; 94:1236–43.
11. Yabe D, Seino Y, Fukushima M, Seino S. β Cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015; 15:602.

12. Qian L, Xu L, Wang X, Fu X, Gu Y, Lin F, et al. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev. 2009; 25:144–9.

13. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001; 50:590–3.

14. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003; 88:2300–8.
15. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–40.
16. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002; 3:141–6.

17. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006; 368:1681–8.

18. Matthews DR, Paldanius PM, Proot P, Foley JE, Stumvoll M, Del Prato S. Baseline characteristics in the VERIFY study: a randomized trial assessing the durability of glycaemic control with early vildagliptin-metformin combination in newly diagnosed type 2 diabetes. Diabet Med. 2019; 36:505–13.