Abstract
We used serial rectal swabs to investigate the amount and duration of virus secretion through the gastrointestinal tract and assessed the association between fecal shedding and gastrointestinal symptoms and to clarify the clinical usefulness testing rectal swabs. We enrolled ten adult patients hospitalized with symptomatic coronavirus disease 2019 (COVID-19). Respiratory and stool specimens were collected by physicians. The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was confirmed using real-time reverse-transcription polymerase chain reaction. All ten patients had respiratory symptoms, six had diarrhea, and seven were positive for SARS-CoV-2 on rectal swabs. The viral loads in the respiratory specimens was higher than those in the rectal specimens, and no rectal specimens were positive after the respiratory specimens became negative. There was no association between gastrointestinal symptoms, pneumonia, severity, and rectal viral load. Rectal swabs may play a role in detecting SARS-CoV-2 in individuals with suspected COVID-19, regardless of gastrointestinal symptoms.
Graphical Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is global epidemic in many countries, including South Korea. Although most COVID-19 patients have fever and respiratory symptoms and SARS-CoV-2 is usually transmitted by personal contact with a case with these symptoms,1 there are a few studies showing that SARS-CoV-2 infection can be accompanied by gastrointestinal (GI) symptoms and can be transmitted by fecal-oral transmission.234 A recent Korean study showed that the viral load could be checked in fecal samples of patients with asymptomatic and mild COVID-19 who were admitted to a residential treatment center.5 In addition, SARS-CoV-2 viral load could be also detected in the saliva in two Korean studies.67 The main GI symptoms of COVID-19 are diarrhea, nausea, vomiting, and abdominal pain. In particular, the prevalence of diarrhea has been reported to range from 2.0% to 34.8%.8910 A recent Korean study also revealed that 13% of COVID-19 patients had GI symptoms such as nausea, vomiting or diarrhea.11 The diagnosis of COVID-19 involves the detection of SARS-CoV-2 using real-time reverse-transcription polymerase chain reaction (rRT-PCR) in respiratory samples. Although it is thought that SARS-CoV-2 can be transmitted through the fecal-oral route, there is a lack of information on the amount of viral shedding through the GI tract. Furthermore, there is little evidence of an association between viral shedding in the GI tract and symptoms. In this study, we investigated the amount and duration viral shedding using serial rectal swabs to investigate the possibility of fecal-oral transmission of SARS-CoV-2 and to determine whether GI shedding is associated with symptoms.
We enrolled ten adult patients who were hospitalized for symptomatic COVID-19. All patients were interviewed by physicians about their respiratory and gastrointestinal symptoms. Respiratory and stool specimens were collected three times a week by physicians using polyester flocked swabs. We confirmed the presence of SARS-CoV-2 in these specimens using rRT-PCR (PowerCheck™ 2019-nCoV Real-time PCR Kit; KogeneBiotech, Seoul, Korea). Samples with cycle threshold (Ct) values exceeding 40 cycles were defined as negative.
The significance of differences in characteristics according to the participant characteristics was determined using Fisher's exact test for categorical variables, and the Mann-Whitney U test for continuous variables. P values ≤ 0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) ver. 18.0 (SPSS, Chicago, IL, USA).
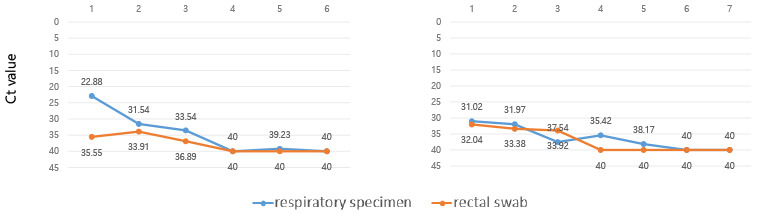
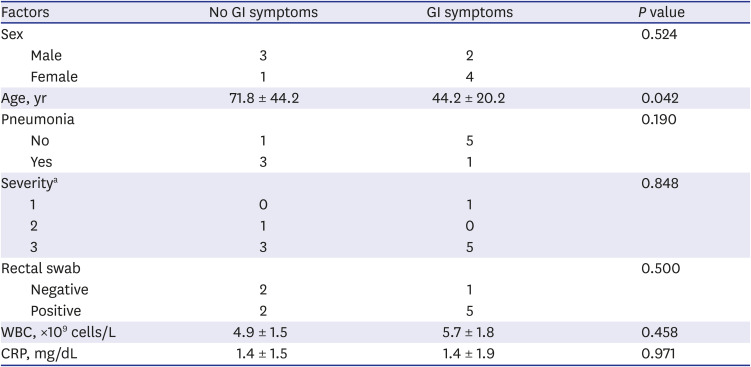
Ten consecutive patients were enrolled in this study. Their characteristics are shown in Table 1. Their mean age was 55.2 ± 21.9 years and five (50%) were male. Radiologic examination, including chest computed tomography, confirmed pneumonia in 4 patients. Eight patients complained of mild symptoms and received conservative treatment, and only two patients received oxygen supplement therapy, and one of them required mechanical ventilation. Six patients had gastrointestinal symptoms. All patients with gastrointestinal symptoms had diarrhea. Patients with gastrointestinal symptoms were significantly younger than patients without gastrointestinal symptoms (mean age 44.2 ± 20.2 years and 71.8 ± 44.2 years, respectively, P = 0.042) (Table 2). Fig. 1 shows the serial viral load from respiratory and stool specimens by Ct values from rRT-PCR during hospitalization in all 10 cases. SARS-CoV-2 was detected in both respiratory and rectal swab specimens of 7 patients. In all rRT-PCR results, except for one result in case 4, the viral load of the respiratory specimen was higher than that of the rectal specimen, based on the Ct values. However, there was no significant association between gastrointestinal symptoms, pneumonia, severity, and rectal viral load. Table 2 shows the clinical features according to the presence or absence of GI symptoms.
These results show that COVID-19 could spread through the GI tract. In addition, we found that as the viral load in respiratory specimens decreases, the viral load in rectal specimens also decreases. This finding is similar to that of an earlier study that reported that the viral load of the respiratory specimens appears to be related to fecal shedding of the virus.5 However, a few studies have shown that rectal swabs are more likely than oral swabs to be positive in the late stages of COVID-19.1213 This difference is presumed to be due to the difference in the sampling methods and viral load measurement methods. A limitation of this study is its small sample size; however, unlike other studies, we reduced contamination of test results as much as possible by having physicians collect the rectal swabs and measured viral load by rRT-PCR for accuracy. Although there are several studies related to GI symptoms in COVID-19 infection, the association between the presence of GI symptoms and the prognosis showed contradictory results.9141516 This prospective study pointed out that 60% of patients experienced GI symptoms, of which diarrhea was the main symptom. However, we did not find any association between GI symptoms, pneumonia severity, and viral load in rectal swabs. Considering that most previous studies were retrospective studies that relied on medical records, a large prospective study that clearly defines GI symptoms and rectal viral load is needed.
Notably, we found that younger patients were more likely than older patients to have GI symptoms. Therefore, COVID-19 should be considered a differential diagnosis of younger patients with GI symptoms in areas where COVID-19 is prevalent. The present study showed that in 7 of 10 patients, SARS-CoV-2 was present simultaneously in the respiratory and GI tracts, indicating that patients may have concurrent respiratory and GI infections. Because COVID-19 is a respiratory disease, it is easy to focus only on symptoms such as cough or sputum. However, GI symptoms, such as diarrhea, should be checked. Compared to respiratory infections, the viral load found in rectal swabs or stool was relatively low, but caution is needed because there is a possibility of transmission via the feco-oral route.
Some patients were uncomfortable and reluctant to participate in a study that involved the collection of rectal swab or stool samples, so several patients refused to participate. In addition, as the viral load detected in the rectal swab and stool samples was lower than that of the respiratory specimens, rectal swabs may not be suitable for use in screening for infection. Rectal swabs can be performed in the following cases: First, if a young patient with diarrhea accompanied by fever has a negative SARS-CoV-2 rRT-PCR test on a respiratory sample, a test could be performed on a rectal swab or stool sample. Second, it could help with the decision to discharge patients with diarrhea when both the respiratory and stool test results are negative. This could reduce the incidence of community-acquired infections. Even without respiratory symptoms, patients with SARS-CoV-2 in the GI tract could be a major source of infection in the community.
In conclusion, this is the first SARS-CoV-2 screening study involving rectal swabs in hospitalized symptomatic COVID-19 Korean patients. Fecal-oral transmission of SARS-CoV-2 is possible with or without GI symptoms. Therefore, rectal swabs may play a role in testing for SARS-CoV-2 in individuals in whom COVID-19 is suspected, regardless of GI symptoms and determining the potential risk of COVID-19.
References
1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223):507–513. PMID: 32007143.

2. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020; 158(6):1831–1833.e3. PMID: 32142773.

3. D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020; 18(8):1663–1672. PMID: 32278065.
4. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020; 5(4):335–337. PMID: 32087098.

5. Park SK, Lee CW, Park DI, Woo HY, Cheong HS, Shin HC, et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin Gastroenterol Hepatol. 2021; 19(7):1387–1394.e2. PMID: 32534042.

6. Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020; 35(20):e195. PMID: 32449329.

7. Kim SE, Lee JY, Lee A, Kim S, Park KH, Jung SI, et al. Viral load kinetics of SARS-CoV-2 infection in saliva in Korean patients: a prospective multi-center comparative study. J Korean Med Sci. 2020; 35(31):e287. PMID: 32776725.

8. Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020; 69(6):997–1001. PMID: 32241899.

9. Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020; 159(1):373–375.e2. PMID: 32294477.
10. Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020; 159(2):765–767.e2. PMID: 32333911.

11. Jang SY, Seon JY, Eun BL, Koh SB, Yoo JH, Lee WY, et al. Risk factors of outcomes of COVID-19 patients in Korea: focus on early symptoms. J Korean Med Sci. 2021; 36(18):e132. PMID: 33975399.

12. Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020; 9(1):386–389. PMID: 32065057.

13. Nouri-Vaskeh M, Alizadeh L. Fecal transmission in COVID-19: a potential shedding route. J Med Virol. 2020; 92(10):1731–1732. PMID: 32239515.

14. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708–1720. PMID: 32109013.

15. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061–1069. PMID: 32031570.

16. Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020; 69(6):1002–1009. PMID: 32213556.
Fig. 1
The serial viral load from respiratory and stool specimens by threshold cycle values from real-time reverse-transcription polymerase chain reaction in all 10 cases.
Ct = cycle threshold.
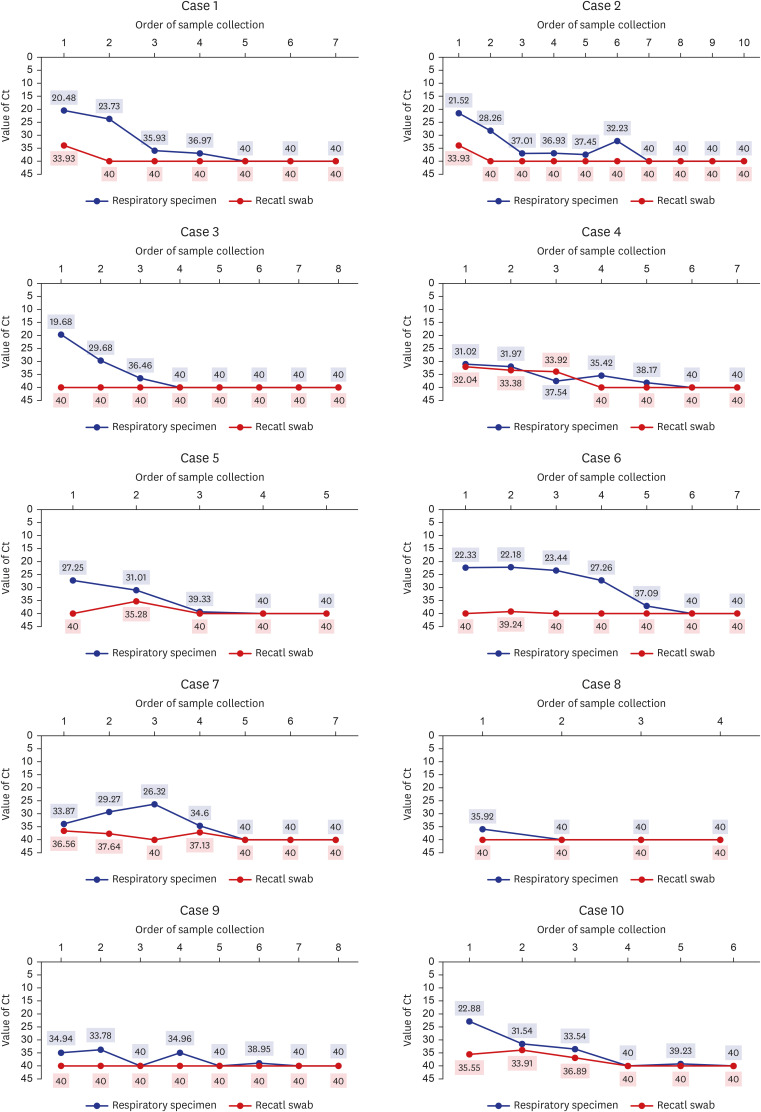
Table 1
Demographics of COVID-19 patients with or without gastrointestinal symptoms
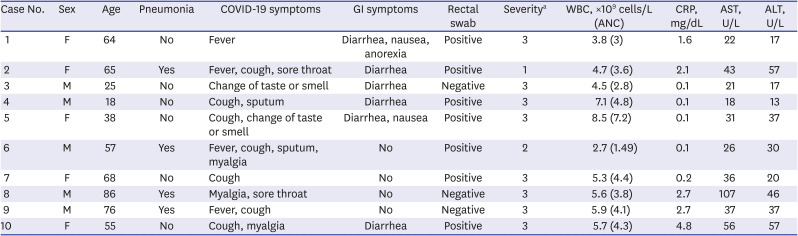
COVID-19 = coronavirus disease 2019, GI = gastrointestinal, WBC = white blood cells, ANC = absolute neutrophil count, CRP = C-reactive protein, AST = aspartate aminotransferase, ALT = alanine aminotransferase.
aSeverity: 1. mechanical ventilator or high oxygen therapy, 2. oxygen therapy, 3. conservative care.
Table 2
Factors associated with the presence of gastrointestinal symptoms




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download