INTRODUCTION
When predicting growth and planning orthodontic and/or orthopedic treatments in growing patients, clinicians must consider diverse craniofacial skeletal and dental characteristics. Several growth prediction methods have provided information on whether orthodontic and/or orthopedic treatments can be applied or should be delayed until the completion of growth by using patient or population-based cephalometric data.
1-8 In particular, Ricketts
1,2,4 studied several cephalometric growth prediction methods to determine mandibular growth. Barbosa et al.
8 also compared longitudinal growth changes between patients with Class I and Class II division 2 malocclusions by using lateral cephalograms. However, the influences of genetic and environmental factors on skeletal, dental, and soft tissue characteristics cannot be completely investigated using simple cephalometric analysis. Therefore, genetic analyses have been performed using parent-offspring correlation, model fitting, questionnaire with pedigree chart, and twin model studies.
2-7
Monozygotic (MZ) twins share identical genetic information, whereas dizygotic (DZ) twins share only half of their genetic information. Therefore, genetic differences between MZ and DZ twins can be determined under an assumption of identical environments for the MZ and DZ twins. Classical twin study designs, including the ACE model and Falconer’s formula, are useful for examining the effects of genetic and environmental variances on phenotypic expression.
9-11 Therefore, numerous twin studies
12-22 have included both MZ and DZ twins.
The capability of twin studies to accurately analyze the effects of genetic and environmental factors on the sizes and shapes of craniofacial structures has been utilized by numerous previous studies.
12-22 Nevertheless, several factors must be considered when designing the twin studies. First, the degree of heritability estimates can be influenced by the age of the subjects. If the subjects are adolescents, their mandibular growth will continue until the completion of growth. Therefore, twin pairs under the age of 19 years should be excluded to minimize the influence of age. Second, the sex of DZ twin pairs should be matched to minimize errors arising from the differences in cephalometric linear and angular variables between males and females.
20-22
Although some studies have investigated the influences of genetic and environmental factors on craniofacial morphology using adult twins,
20-22 no twin study to date compared the craniofacial skeletal and dental characteristics between twin pairs with skeletal Class I and Class II malocclusions. Therefore, the purpose of this study was to investigate the differences in the heritability of craniofacial skeletal and dental characteristics between MZ and DZ twin pairs with skeletal Class I and Class II malocclusions.
Go to :

MATERIALS AND METHODS
The initial sample included 126 Korean adult twins (48 MZ and 15 DZ twin pairs) whose lateral cephalograms were available at the Samsung Medical Center, Seoul, Republic of Korea. The study protocol was reviewed and approved by the Institutional Review Board of the School of Public Health, Seoul National University, Seoul, Republic of Korea (IRB 2005-08-113-027). Informed consent was obtained from all subjects.
The inclusion criteria were as follows: (1) MZ or DZ twin pairs, (2) DZ twin pairs with the same sex, (3) age over 19 years, and (4) a skeletal Class I or Class II pattern (angle between point A, nasion, and point B [ANB] > 0°). These criteria were employed to avoid any bias from age and sex.
20-22
The exclusion criteria were as follows: (1) an edentulous area of the anterior teeth, (2) use of a removable prosthesis, and (3) a history of orthodontic treatment or orthognathic surgery. These criteria were employed to avoid the influences of these conditions on the profile and vertical dimension of the face.
20-22
As the final sample, 40 Korean adult MZ and DZ twin pairs (mean age, 41.9 ± 8.3 years; 40 males and 40 females) were selected. They were divided into the Class I group (n = 20 twin pairs; 0° ≤ ANB ≤ 4°; mean age, 40.7 ± 7.4 years) and the Class II group (n = 20 twin pairs; ANB > 4°; mean age, 43.0 ± 9.0 years). Each group comprised 14 MZ and 6 DZ twin pairs with the same sex (20 males and 20 females per group;
Table 1).
Table 1
Demographic data of samples
|
Variable |
Class I group |
Class II group |
Significance |
|
Distribution of pairs |
14 MZ pairs and 6 DZ pairs |
14 MZ pairs and 6 DZ pairs |
1.0000 |
|
Sex |
20 males and 20 females |
20 males and 20 females |
1.0000 |
|
Age (yr) |
40.71 ± 7.36 |
43.02 ± 9.03 |
0.2582 |
|
ANB (°) |
2.10 ± 1.26 |
5.04 ± 1.47 |
0.0001*** |

The landmarks and reference lines used for cephalometric analysis are illustrated in
Figure 1. The craniofacial characteristics were categorized into the anteroposterior (AP), vertical, dental, mandibular and cranial base characteristics for investigating the areas influenced by heredity.
20-22 The linear, angular, and ratio variables, which could describe the sizes and shapes of these structures (
Figure 2), were measured by a single operator (EMK) using the V-Ceph 6.0 program (Cybermed, Seoul, Korea). Since overbite depth indicator showed significant differences between the MZ and DZ twin subgroups (
p < 0.01 in the Class I group,
p < 0.05 in the Class II group) and lower gonial angle showed significant differences between the MZ and DZ twin subgroups (
p < 0.05 in the Class I group;
Table 2), these variables were excluded. Finally, 33 cephalometric variables were selected for further investigation (
Table 2).
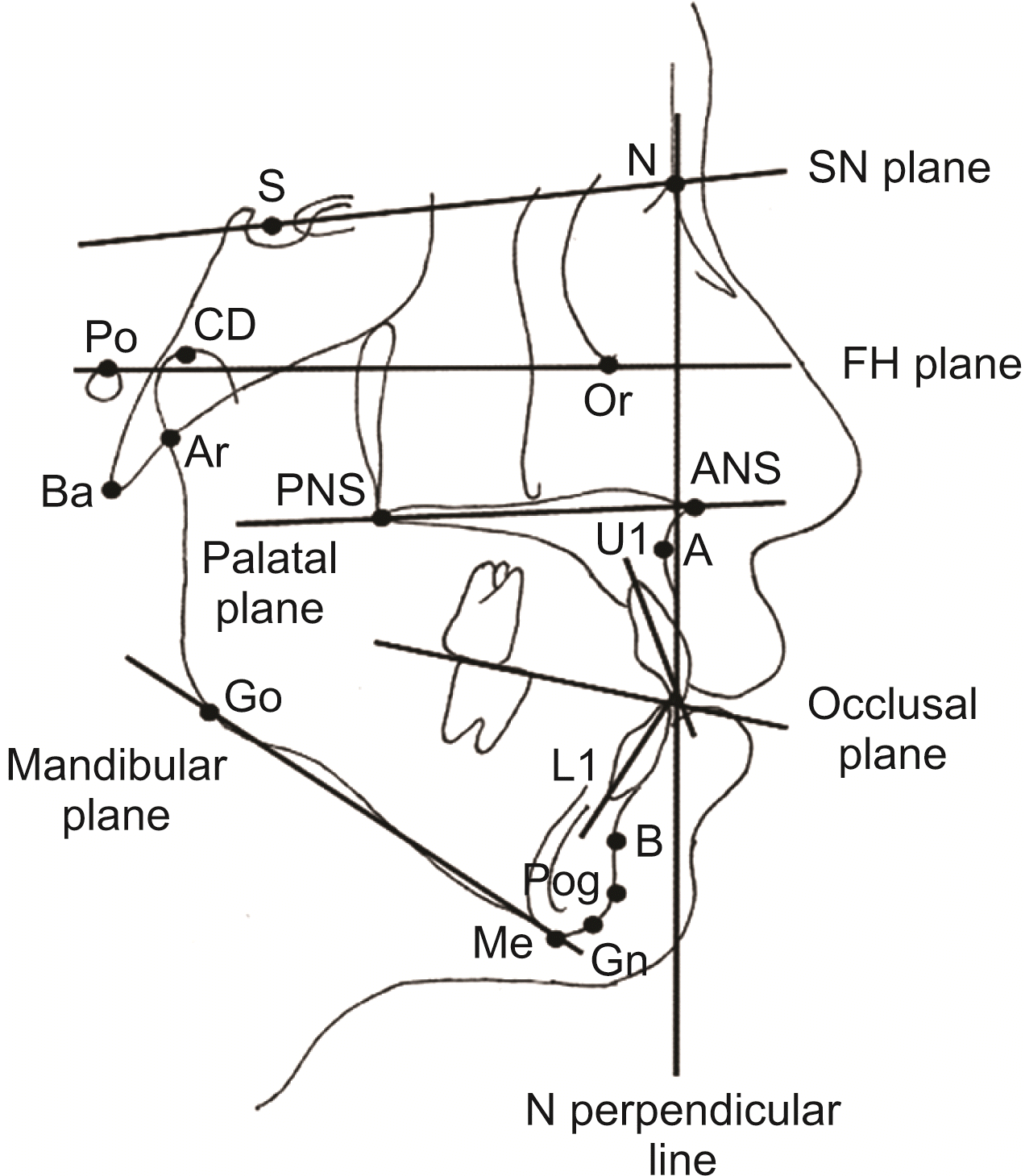 | Figure 1
Landmarks and reference lines used in the cephalometric analysis.
Landmarks: S, sella; N, nasion; Po, porion; Or, orbitale; CD, condylion; Ar, articulare; Ba, basion; PNS, posterior nasal spine; ANS, anterior nasal spine; A, A point; B, B point; Pog, pogonion; Gn, gnathion; Me, menton; Go, gonion; Reference lines: SN plane; Frankfort-horizontal (FH) plane; palatal plane (PP); occlusal plane (OP); mandibular plane (MP); N perpendicular line; U1, long axis of the upper incisor; L1, long axis of the lower incisor.

|
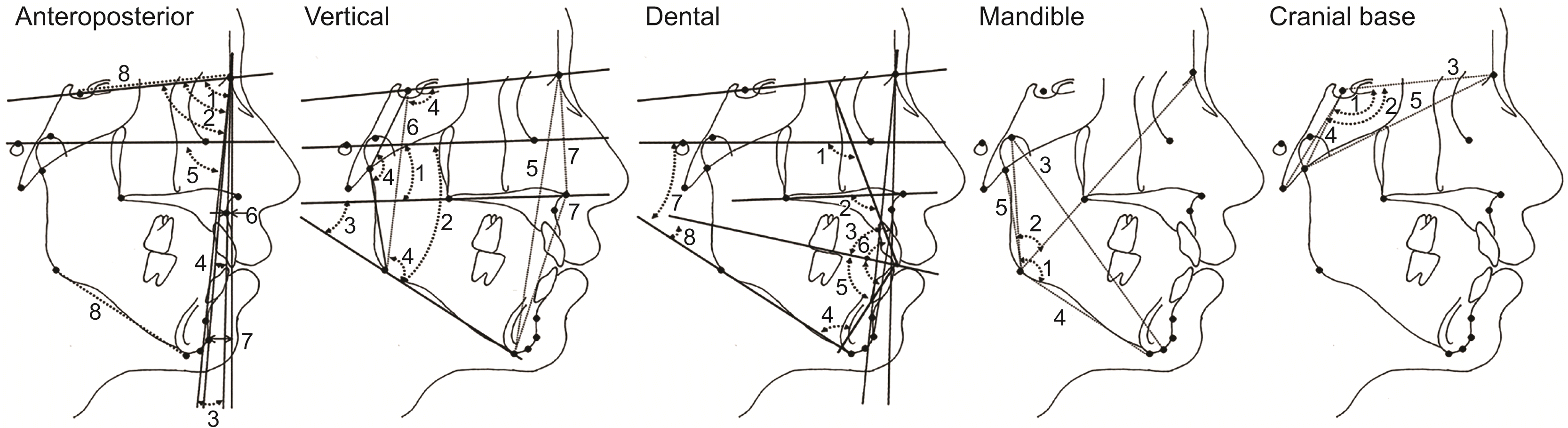 | Figure 2
Cephalometric variables.
Anteroposterior characteristics: 1, SNA (angle between S, N, and point A); 2, SNB (angle between S, N, and point B); 3, ANB (angle between point A, N, and point B); 4, NA-Pog (angle between N, point A, and Pog); 5, FH-NPog (angle formed by FH plane and N-Pog line); 6, A-N perpendicular (perpendicular distance from point A to the N perpendicular line); 7, Pog-N perpendicular (perpendicular distance from Pog to the N perpendicular line); and 8, mandibular body length/anterior cranial base (ratio of the distance between Go and Me to the distance between S and N). Vertical characteristics: 1, FH-PP (angle formed by FH plane and palatal plane); 2, FMA (angle formed by FH plane and mandibular plane); 3, PP-MP (angle formed by PP and MP); 4, Bjork sum (summation of angles determined by saddle angle, articular angle, and gonial angle); 5, N-Me (distance between N and Me); 6, S-Go (distance between S and Go); and 7, N-ANS/ANS-Me (ratio of the distance between N and ANS to the distance between ANS and Me). Dental characteristics: 1, U1-FH (angle formed by upper incisor axis and FH plane); 2, U1-PP (angle formed by U1 and PP); 3, U1-OP (angle formed by U1 and OP); 4, IMPA (angle formed by lower incisor axis and MP); 5, L1-OP (angle formed by L1 and OP); 6, interincisal angle (angle formed by U1 and L1); 7, FH-OP (angle formed by FH plane and OP); and 8, OP-MP (angle formed by OP and MP). Mandible characteristics: 1, gonial angle (angle between Ar, Go, and Me); 2, upper gonial angle (angle between Ar, Go, and N); 3, CD-Gn (distance between condyle head and Gn); 4, Go-Me (distance between Go and Me); and 5, Ar-Go (distance between Ar and Go). Cranial base characteristics: 1, saddle angle (angle between N, S, and Ar); 2, cranial base angle (angle between N, S, and Ba); 3, S-N (distance between S and N); 4, S-Ar (distance between S and Ar); and 5, Ar-N (distance between Ar and N).
See Figure 1 for definition of each landmark. 
|
Table 2
Comparison of cephalometric variables measured between the monozygotic (MZ) and dizygotic (DZ) twin subgroups within Class I and Class II groups
|
Cephalometric variable |
Class I group |
|
Class II group |
Class I-DZ
(n = 6 pairs) |
|
Class I-MZ
(n = 14 pairs) |
p-value |
Class II-DZ
(n = 6 pairs) |
|
Class II-MZ
(n = 14 pairs) |
p-value |
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
Antero-posterior |
SNA (°) |
80.43 |
2.52 |
|
81.16 |
3.91 |
0.5354 |
|
83.96 |
4.40 |
|
81.45 |
3.00 |
0.1023 |
|
SNB (°) |
78.51 |
3.01 |
|
78.99 |
3.70 |
0.6687 |
|
78.81 |
4.47 |
|
76.47 |
2.89 |
0.1365 |
|
ANB (°) |
1.94 |
1.57 |
|
2.17 |
1.13 |
0.7454 |
|
5.16 |
1.40 |
|
4.99 |
1.51 |
0.5710 |
|
Facial convexity angle (NA-Pog, °) |
177.86 |
6.74 |
|
177.40 |
3.68 |
0.9647 |
|
170.16 |
3.85 |
|
170.67 |
3.42 |
0.6542 |
|
Facial angle (FH-NPog, °) |
89.25 |
3.14 |
|
88.56 |
2.71 |
0.3301 |
|
87.21 |
2.10 |
|
86.66 |
2.33 |
0.1200 |
|
A-N perpendicular (mm) |
0.36 |
2.54 |
|
–0.20 |
3.01 |
0.3525 |
|
2.01 |
1.63 |
|
1.18 |
2.45 |
0.1023 |
|
Pog-N perpendicular (mm) |
4.45 |
4.73 |
|
5.09 |
3.30 |
0.2814 |
|
6.34 |
2.92 |
|
7.21 |
3.71 |
0.1509 |
|
Mandibular body length/
anterior cranial base (Go-Me/S-N) |
1.16 |
0.09 |
|
1.13 |
0.08 |
0.4517 |
|
1.10 |
0.10 |
|
1.10 |
0.06 |
0.8536 |
|
Vertical |
ODI |
67.02 |
6.76 |
|
73.14 |
3.59 |
0.0048** |
|
74.71 |
5.96 |
|
77.17 |
5.32 |
0.0362* |
|
FH-PP (°) |
0.07 |
3.46 |
|
1.08 |
2.58 |
0.4517 |
|
1.28 |
3.74 |
|
1.59 |
2.31 |
0.6829 |
|
FMA (°) |
27.11 |
3.82 |
|
23.62 |
4.97 |
0.0570 |
|
27.06 |
5.49 |
|
25.82 |
5.66 |
0.3917 |
|
PP-MP (°) |
25.36 |
4.91 |
|
22.55 |
4.41 |
0.1213 |
|
25.78 |
5.54 |
|
24.22 |
6.42 |
0.2204 |
|
Bjork sum (°) |
395.33 |
2.56 |
|
392.27 |
5.62 |
0.0954 |
|
395.07 |
6.80 |
|
395.43 |
5.99 |
0.7419 |
|
Anterior facial height (AFH, N-Me, mm) |
122.34 |
4.90 |
|
120.66 |
6.21 |
0.3375 |
|
120.53 |
5.90 |
|
121.61 |
7.18 |
0.5532 |
|
Posterior facial height (PFH, S-Go, mm) |
80.52 |
4.76 |
|
82.59 |
8.47 |
0.6263 |
|
80.80 |
6.16 |
|
80.73 |
7.87 |
0.6637 |
|
N-ANS/ANS-Me |
0.78 |
0.07 |
|
0.81 |
0.05 |
0.1481 |
|
0.79 |
0.06 |
|
0.85 |
0.09 |
0.0561 |
|
Dental |
U1-FH (°) |
116.64 |
6.63 |
|
113.25 |
6.35 |
0.0570 |
|
114.19 |
5.51 |
|
111.79 |
6.81 |
0.2012 |
|
U1-PP (°) |
118.38 |
7.91 |
|
114.33 |
6.42 |
0.0742 |
|
115.47 |
5.87 |
|
113.40 |
6.49 |
0.3991 |
|
U1-OP (°) |
54.20 |
3.94 |
|
55.74 |
4.99 |
0.1793 |
|
54.26 |
6.75 |
|
56.30 |
6.02 |
0.2517 |
|
IMPA (°) |
92.98 |
7.57 |
|
97.02 |
7.59 |
0.1213 |
|
100.19 |
5.98 |
|
100.57 |
5.92 |
0.4686 |
|
L1-OP (°) |
67.91 |
6.34 |
|
68.25 |
8.51 |
0.5257 |
|
61.65 |
5.66 |
|
63.03 |
7.60 |
0.8228 |
|
Interincisal angle (°) |
121.60 |
10.05 |
|
126.11 |
11.05 |
0.1891 |
|
118.57 |
10.02 |
|
121.81 |
11.43 |
0.5016 |
|
FH-OP (°) |
9.16 |
4.83 |
|
11.01 |
3.09 |
0.0514 |
|
11.56 |
4.30 |
|
11.90 |
5.15 |
0.7419 |
|
OP-MP (°) |
16.83 |
4.16 |
|
13.77 |
5.16 |
0.0742 |
|
16.20 |
3.39 |
|
14.29 |
5.27 |
0.1665 |
|
Mandible |
Gonial angle (Ar-Go-Gn, °) |
122.38 |
8.79 |
|
118.45 |
7.51 |
0.2097 |
|
121.11 |
8.02 |
|
119.33 |
5.59 |
0.2409 |
|
Upper gonial angle (Ar-Go-N, °) |
44.46 |
4.26 |
|
44.91 |
3.90 |
0.8943 |
|
43.87 |
4.61 |
|
44.06 |
2.31 |
0.6829 |
|
Lower gonial angle (°) |
77.92 |
5.00 |
|
73.54 |
4.43 |
0.0126*
|
|
77.23 |
5.80 |
|
75.26 |
4.85 |
0.1232 |
|
CD-Gn (mm) |
108.62 |
8.34 |
|
107.44 |
8.53 |
0.7566 |
|
103.28 |
8.43 |
|
104.89 |
9.45 |
0.4768 |
|
Go-Me (mm) |
73.74 |
3.96 |
|
72.98 |
5.61 |
0.5851 |
|
69.23 |
5.67 |
|
70.80 |
4.65 |
0.4850 |
|
Ar-Go (mm) |
48.01 |
5.20 |
|
50.67 |
7.06 |
0.1992 |
|
47.44 |
4.78 |
|
48.56 |
6.36 |
0.2979 |
|
Cranial base |
Saddle angle (N-S-Ar, °) |
126.95 |
4.13 |
|
127.34 |
5.32 |
0.8479 |
|
123.72 |
3.16 |
|
125.89 |
4.14 |
0.0820 |
|
Cranial base angle (N-S-Ba, °) |
133.64 |
4.03 |
|
133.56 |
4.78 |
0.7343 |
|
131.58 |
5.10 |
|
133.07 |
3.57 |
0.4216 |
|
S-N (mm) |
62.23 |
3.37 |
|
64.61 |
2.45 |
0.1523 |
|
62.97 |
3.35 |
|
64.25 |
3.37 |
0.1547 |
|
S-Ar (mm) |
35.91 |
2.99 |
|
35.74 |
3.26 |
0.6159 |
|
35.98 |
2.98 |
|
35.41 |
3.41 |
0.8023 |
|
Ar-N (mm) |
89.22 |
4.50 |
|
90.80 |
4.55 |
0.2097 |
|
87.70 |
4.39 |
|
90.44 |
5.15 |
0.0546 |

All variables from 20 randomly selected subjects were remeasured by the same operator (EMK) after a 2-week interval. The intra-operator measurement error was assessed using the intraclass correlation coefficient (ICC). Since no significant differences were observed between the first and second measurements, the first set of measurements was used for anlaysis.
The genetic effect (A) on MZ twins is equal because they have identical genetic information; however, DZ twins with the same sex share only half of their genetic information. In addition, both MZ and DZ twins are assumed to have the same environmental effect (E).
20-24 Therefore, Pearson’s correlation coefficients (r
mz, r
dz), i.e., the sum of the genetic and environmental effects on the phenotype, were calculated as follows: r
mz = A + E in MZ twin pairs and r
dz =
12 / A + E in DZ twin pairs (
Table 3).
Table 3
The effect of genetic and environmental factors on the facial anteroposterior, facial vertical, dental, mandibular, and cranial base variables measured in the Class I and Class II groups
|
Cephalometric variable |
Class I group |
|
Class II group |
|
rmz
|
rdz
|
rmz
|
rdz
|
|
Facial anteroposterior |
SNA (°) |
0.8734 |
0.2669 |
|
0.7971 |
0.7297 |
|
SNB (°) |
0.9093 |
0.1711 |
|
0.8475 |
0.5774 |
|
ANB (°) |
0.3208 |
−0.3771 |
|
0.5265 |
0.0237 |
|
Facial convexity angle (NA-Pog, °) |
0.6272 |
−0.5416 |
|
0.5662 |
0.5351 |
|
Facial angle (FH-NPog, °) |
0.8499 |
0.5911 |
|
0.8230 |
0.1797 |
|
A-N perpendicular (mm) |
0.7177 |
0.3745 |
|
0.6063 |
−0.2908 |
|
Pog-N perpendicular (mm) |
0.7767 |
0.2050 |
|
0.7055 |
0.2208 |
|
Mandibular body length/
anterior cranial base (Go-Me/S-N) |
0.8140 |
0.0793 |
|
0.6307 |
0.9211 |
|
Facial vertical |
FH-PP (°) |
0.7236 |
−0.2690 |
|
0.5624 |
0.8477 |
|
FMA (°) |
0.7737 |
0.6259 |
|
0.8516 |
0.6647 |
|
PP-MP (°) |
0.7947 |
−0.4834 |
|
0.8705 |
0.5104 |
|
Bjork sum (°) |
0.8367 |
−0.2503 |
|
0.8772 |
0.7987 |
|
Anterior facial height (AFH, N-Me, mm) |
0.9343 |
0.8561 |
|
0.9386 |
0.0557 |
|
Posterior facial height (PFH, S-Go, mm) |
0.8937 |
0.8670 |
|
0.9459 |
0.5514 |
|
N-ANS/ANS-Me |
0.6224 |
0.4323 |
|
0.8858 |
0.5315 |
|
Dental |
U1-FH (°) |
0.8424 |
−0.5742 |
|
0.6243 |
−0.0880 |
|
U1-PP (°) |
0.7284 |
−0.5928 |
|
0.6274 |
0.0784 |
|
U1-OP (°) |
0.6576 |
0.2775 |
|
0.5073 |
0.7615 |
|
IMPA (°) |
0.8583 |
−0.0917 |
|
0.6892 |
0.2079 |
|
L1-OP (°) |
0.7144 |
0.6386 |
|
0.5480 |
−0.6280 |
|
Interincisal angle (°) |
0.7618 |
0.1741 |
|
0.7709 |
0.7106 |
|
FH-OP (°) |
0.5391 |
−0.2724 |
|
0.8685 |
0.5846 |
|
OP-MP (°) |
0.2113 |
0.5529 |
|
0.6989 |
−0.1669 |
|
Mandible |
Gonial angle (Ar-Go-Gn, °) |
0.7241 |
0.6508 |
|
0.5764 |
0.3033 |
|
Upper gonial angle (Ar-Go-N, °) |
0.4816 |
0.6336 |
|
0.2193 |
−0.1697 |
|
CD-Gn (mm) |
0.2279 |
0.6605 |
|
0.8234 |
0.3373 |
|
Go-Me (mm) |
0.8756 |
−0.5329 |
|
0.7662 |
0.8101 |
|
Ar-Go (mm) |
0.8455 |
0.8664 |
|
0.9072 |
0.5218 |
|
Cranial base |
Saddle angle (N-S-Ar, °) |
0.7366 |
0.0286 |
|
0.7372 |
0.7375 |
|
Cranial base angle (N-S-Ba, °) |
0.9093 |
−0.1470 |
|
0.7255 |
0.9811 |
|
S-N (mm) |
0.9184 |
0.9953 |
|
0.8933 |
0.4504 |
|
S-Ar (mm) |
0.9351 |
0.7824 |
|
0.8194 |
0.6911 |
|
Ar-N (mm) |
0.9241 |
0.7717 |
|
0.9635 |
0.5517 |

Based on the difference between the correlation coefficients for MZ twin pairs and DZ twin pairs with the same sex, the ACE model was used to calculate the additive genetic effects (A), common environmental effects (C), and specific environmental effects (E).
20 This provided information about the heritability (A) of twins.
23 In the present study, an A value above 0.7 was considered to indicate high heritability and an A value between 0.4 and 0.7 was considered to indicate moderate heritability.
Principal component analysis (PCA) with Kaiser normalization varimax rotation was used to extract components by grouping the cephalometric variables in the Class I and Class II groups.
21,22,25 The components with an eigenvalue higher than 1 were chosen.
18,19 After the mean ICC values of each component were calculated, the A value was also calculated for the Class I and Class II groups.
21,22
All statistical analyses were performed using SPSS program version 21.0 (IBM Corp., Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant.
Go to :

RESULTS
Genetic heritability (A) in the Class I group (Table 4)
Among the AP characteristics, the maxilla, mandible, and intermaxillary relationship showed high A values (SNA, 0.80; SNB, 0.86; facial convexity angle, 0.74; facial angle, 0.74; Pog-N perpendicular, 0.84; and Go-Me/S-N, 0.70). Among the vertical characteristics, two angular variables showed high A values (FH-PP, 0.74; PP-MP, 0.74). Among the dental characteristics, the inclination of the maxillary and mandibular incisors and interincisal angle showed high A values (U1-FH, 0.82; U1-PP, 0.73; IMPA, 0.87; and interincisal angle, 0.75). Among the mandibular characteristics, only mandibular body length showed a high A value (Go-Me, 0.75). Among the cranial base characteristics, cranial base angle showed a high A value (N-S-Ba, 0.86).
Table 4
Genetic effects (A), common environmental effects (C), and specific environmental effects (E) of the facial anteroposterior, facial vertical, dental, mandibular, and cranial base structures in the Class I and Class II groups
|
Variable |
Class I group |
|
Class II group |
|
A |
C |
E |
A |
C |
E |
|
Facial anteroposterior |
SNA (°) |
0.7982*
|
0.0000 |
0.2018 |
|
0.4498 |
0.3955 |
0.1547 |
|
SNB (°) |
0.8590*
|
0.0000 |
0.1410 |
|
0.8931*
|
0.0000 |
0.1069 |
|
ANB (°) |
0.1321 |
0.0000 |
0.8679 |
|
0.4257 |
0.0000 |
0.5743 |
|
Facial convexity angle (NA-Pog, °) |
0.7367*
|
0.0000 |
0.2633 |
|
0.1765 |
0.3454 |
0.4781 |
|
Facial angle (FH-NPog, °) |
0.7360*
|
0.1087 |
0.1553 |
|
0.8042*
|
0.0000 |
0.1958 |
|
A-N perpendicular (mm) |
0.6615 |
0.0000 |
0.3385 |
|
0.4757 |
0.0000 |
0.5243 |
|
Pog-N perpendicular (mm) |
0.8358*
|
0.0000 |
0.1642 |
|
0.6434 |
0.0000 |
0.3566 |
|
Mandibular body length/
anterior cranial base (Go-Me/S-N) |
0.7035*
|
0.0000 |
0.2965 |
|
0.3501 |
0.3118 |
0.3380 |
|
Facial vertical |
FH-PP (°) |
0.7401*
|
0.0000 |
0.2599 |
|
0.0642 |
0.6035 |
0.3323 |
|
FMA (°) |
0.1165 |
0.6352 |
0.2483 |
|
0.6195 |
0.2124 |
0.1681 |
|
PP-MP (°) |
0.7404*
|
0.0000 |
0.2596 |
|
0.8364*
|
0.0000 |
0.1636 |
|
Bjork sum (°) |
0.3087 |
0.4786 |
0.2128 |
|
0.4854 |
0.3729 |
0.1417 |
|
Anterior facial height (AFH, N-Me, mm) |
0.0846 |
0.8404 |
0.0751 |
|
0.9324*
|
0.0000 |
0.0676 |
|
Posterior facial height (PFH, S-Go, mm) |
0.0000 |
0.8540 |
0.1460 |
|
0.9163*
|
0.0000 |
0.0837 |
|
N-ANS/ANS-Me |
0.4987 |
0.0768 |
0.4245 |
|
0.1010 |
0.6641 |
0.2350 |
|
Dental |
U1-FH (°) |
0.8151*
|
0.0000 |
0.1849 |
|
0.5566 |
0.0000 |
0.4434 |
|
U1-PP (°) |
0.7293*
|
0.0000 |
0.2707 |
|
0.5933 |
0.0000 |
0.4067 |
|
U1-OP (°) |
0.1983 |
0.3129 |
0.4889 |
|
0.0000 |
0.5099 |
0.4901 |
|
IMPA (°) |
0.8714*
|
0.0000 |
0.1286 |
|
0.5797 |
0.0000 |
0.4203 |
|
L1-OP (°) |
0.2119 |
0.4638 |
0.3243 |
|
0.4283 |
0.0000 |
0.5717 |
|
Interincisal angle (°) |
0.7493*
|
0.0000 |
0.2507 |
|
0.1168 |
0.6150 |
0.2682 |
|
FH-OP (°) |
0.5430 |
0.0000 |
0.4570 |
|
0.2883 |
0.5207 |
0.1909 |
|
OP-MP (°) |
0.0000 |
0.2587 |
0.7413 |
|
0.6122 |
0.0000 |
0.3878 |
|
Mandible |
Gonial angle (Ar-Go-Gn, °) |
0.3883 |
0.3699 |
0.2418 |
|
0.4909 |
0.0000 |
0.5091 |
|
Upper gonial angle (Ar-Go-N, °) |
0.0000 |
0.5191 |
0.4809 |
|
0.0000 |
0.0000 |
1.0000 |
|
CD-Gn (mm) |
0.0000 |
0.3401 |
0.6599 |
|
0.7422*
|
0.0000 |
0.2578 |
|
Go-Me (mm) |
0.7543*
|
0.0000 |
0.2457 |
|
0.5460 |
0.1999 |
0.2541 |
|
Ar-Go (mm) |
0.0000 |
0.7915 |
0.2085 |
|
0.8067*
|
0.0601 |
0.1332 |
|
Cranial base |
Saddle angle (N-S-Ar, °) |
0.6756 |
0.0000 |
0.3244 |
|
0.0000 |
0.6761 |
0.3239 |
|
Cranial base angle (N-S-Ba, °) |
0.8640*
|
0.0000 |
0.1360 |
|
0.2826 |
0.4946 |
0.2228 |
|
S-N (mm) |
0.0436 |
0.8224 |
0.1340 |
|
0.8392*
|
0.0552 |
0.1056 |
|
S-Ar (mm) |
0.3224 |
0.6076 |
0.0700 |
|
0.8025*
|
0.0000 |
0.1975 |
|
Ar-N (mm) |
0.1854 |
0.6938 |
0.1208 |
|
0.9037*
|
0.0564 |
0.0398 |

Genetic heritability (A) in the Class II group (Table 4)
Among the AP characteristics, only two variables showed high A values (SNB, 0.89 and facial angle, 0.80). Among the vertical characteristics, PP-MP and facial height variables showed high A values (PP-MP, 0.84; anterior facial height, 0.93; and posterior facial height, 0.92). Among the mandibular characteristics, two variables showed high A values (CD-Gn, 0.74 and Ar-Go, 0.81). Among the cranial base characteristics, three variables showed high A values (S-N, 0.84; S-Ar, 0.80; and Ar-N, 0.90). However, none of the dental variables showed high A values.
Comparison of A values between the Class I and Class II groups (Table 4)
Among AP and vertical characteristics, SNB, facial angle, and PP-MP showed high heritability in the two groups. However, none of the dental, mandibular, and cranial base characteristics showed high heritability in the two groups.
PCA in the Class I group (Table 5)
PCA extracted eight components with 88.3% cumulative explanation. Among these eight components, PCA2 and PCA6 had three variables with high A values (U1-PP, U1-FH, and facial angle in PCA2; Go-Me/S-N, Go-Me, and Pog-N perpendicular in PCA6). In addition, PCA4 and PCA5 had two variables with high A values (cranial base angle and SNB in PCA4; IMPA and interincisal angle in PCA5).
Table 5
Principal component analysis (PCA) after varimax rotation in the Class I group with 88.31% explanation
|
Class I group |
PCA1 |
PCA2 |
PCA3 |
PCA4 |
PCA5 |
PCA6 |
PCA7 |
PCA8 |
|
FMA (°) |
0.939 |
−0.146 |
−0.025 |
0.042 |
0.037 |
0.225 |
−0.075 |
0.024 |
|
Bjork sum (°) |
0.848 |
−0.100 |
−0.201 |
0.415 |
−0.003 |
0.029 |
0.022 |
0.082 |
|
PP-MP (°)*
|
0.816 |
−0.259 |
−0.178 |
0.034 |
−0.024 |
0.117 |
−0.027 |
−0.413 |
|
OP-MP (°) |
0.788 |
0.254 |
−0.139 |
−0.096 |
0.003 |
0.302 |
−0.105 |
0.075 |
|
U1-PP (°)*
|
0.031 |
0.930 |
0.138 |
0.085 |
−0.140 |
−0.049 |
−0.083 |
0.152 |
|
U1-FH (°)*
|
−0.083 |
0.927 |
0.040 |
0.084 |
−0.196 |
−0.137 |
−0.053 |
−0.150 |
|
FH-OP (°) |
0.044 |
−0.846 |
−0.050 |
−0.083 |
−0.005 |
−0.093 |
0.021 |
−0.163 |
|
U1-OP (°) |
0.088 |
−0.712 |
−0.028 |
−0.035 |
0.282 |
−0.016 |
0.038 |
0.342 |
|
Facial angle (FH-NPog, °)*
|
−0.481 |
0.550 |
0.000 |
−0.258 |
0.454 |
−0.003 |
0.360 |
−0.099 |
|
Anterior facial height (AFH, N-Me, mm) |
0.079 |
0.104 |
0.946 |
0.136 |
0.045 |
−0.034 |
−0.037 |
0.065 |
|
Ar-N (mm) |
−0.181 |
0.106 |
0.832 |
0.109 |
−0.373 |
0.146 |
−0.074 |
0.028 |
|
S-Ar (mm) |
−0.092 |
−0.079 |
0.809 |
−0.273 |
−0.035 |
−0.267 |
0.108 |
0.117 |
|
Posterior facial height (PFH, S-Go, mm) |
−0.560 |
0.041 |
0.785 |
−0.123 |
0.043 |
−0.015 |
−0.030 |
−0.006 |
|
Ar-Go (mm) |
−0.604 |
0.107 |
0.657 |
0.087 |
0.087 |
0.275 |
−0.071 |
−0.073 |
|
CD-Gn (mm) |
0.095 |
0.172 |
0.597 |
0.108 |
0.053 |
0.495 |
0.265 |
0.105 |
|
Cranial base angle (N-S-Ba, °)*
|
−0.058 |
0.095 |
0.078 |
0.904 |
−0.071 |
0.206 |
0.033 |
−0.065 |
|
Saddle angle (N-S-Ar, °) |
0.009 |
0.187 |
0.327 |
0.846 |
−0.093 |
0.167 |
0.050 |
0.128 |
|
SNB (°)*
|
−0.286 |
0.045 |
0.365 |
−0.781 |
0.147 |
−0.013 |
0.251 |
−0.176 |
|
SNA (°) |
−0.241 |
−0.119 |
0.289 |
−0.722 |
0.039 |
−0.021 |
0.512 |
−0.139 |
|
IMPA (°)*
|
−0.331 |
0.059 |
0.008 |
0.107 |
−0.883 |
−0.048 |
0.097 |
−0.016 |
|
L1-OP (°) |
−0.240 |
−0.259 |
−0.029 |
−0.129 |
0.856 |
−0.179 |
−0.097 |
0.038 |
|
Interincisal angle (°)*
|
−0.176 |
−0.587 |
−0.020 |
−0.148 |
0.730 |
−0.021 |
−0.030 |
0.130 |
|
S-N (mm) |
−0.211 |
0.139 |
0.424 |
−0.103 |
−0.525 |
0.386 |
−0.267 |
−0.133 |
|
Upper gonial angle (Ar-Go-N, °) |
0.329 |
0.023 |
0.141 |
0.123 |
−0.109 |
0.825 |
0.033 |
−0.100 |
Mandibular body length/anterior cranial base
(Go-Me/S-N)*
|
−0.071 |
0.350 |
0.181 |
−0.305 |
0.330 |
−0.730 |
0.223 |
0.026 |
|
Gonial angle (Ar-Go-Gn, °) |
0.624 |
0.001 |
0.140 |
0.139 |
0.095 |
0.717 |
0.032 |
−0.074 |
|
Go-Me (mm) |
−0.245 |
0.496 |
0.372 |
−0.312 |
0.115 |
−0.557 |
0.116 |
−0.026 |
|
Pog-N perpendicular (mm)*
|
0.245 |
−0.255 |
0.209 |
0.340 |
−0.225 |
−0.385 |
−0.205 |
0.179 |
|
A-N perpendicular (mm) |
−0.269 |
0.151 |
0.087 |
−0.330 |
0.057 |
−0.007 |
0.841 |
−0.159 |
|
ANB (°) |
0.111 |
−0.453 |
−0.203 |
0.134 |
−0.283 |
−0.025 |
0.741 |
0.103 |
|
Facial convexity angle (NA-Pog, °)*
|
−0.293 |
0.518 |
−0.095 |
0.077 |
0.512 |
0.006 |
−0.554 |
0.035 |
|
FH-PP (°)*
|
0.123 |
−0.017 |
0.243 |
0.011 |
0.102 |
0.055 |
−0.183 |
0.863 |
|
N-ANS/ANS-Me |
−0.169 |
−0.037 |
−0.076 |
0.186 |
0.009 |
−0.169 |
0.073 |
0.790 |

PCA in the Class II group (Table 6)
PCA extracted seven components with 91.0% cumulative explanation. Among these seven components, PCA1 had seven variables with high A values (anterior facial height, S-N, S-Ar, Ar-Go, CD-Gn, posterior facial height, and Ar-N). PCA2 had two variables with high A values (facial angle and PP-MP).
Table 6
Principal component analysis (PCA) after varimax rotation in the Class II group with 90.99% explanation
|
Class II group |
PCA1 |
PCA2 |
PCA3 |
PCA4 |
PCA5 |
PCA6 |
PCA7 |
|
Bjork sum (°) |
0.977 |
0.006 |
−0.069 |
−0.036 |
0.036 |
0.096 |
0.162 |
|
Anterior facial height (AFH, N-Me, mm)*
|
−0.968 |
−0.116 |
0.114 |
0.100 |
−0.071 |
−0.087 |
−0.078 |
|
Gonial angle (Ar-Go-Gn, °) |
0.961 |
−0.112 |
0.012 |
0.015 |
0.115 |
0.102 |
0.076 |
|
S-N (mm)*
|
−0.950 |
−0.116 |
−0.029 |
0.041 |
−0.144 |
−0.004 |
−0.021 |
|
Saddle angle (N-S-Ar, °) |
0.937 |
0.067 |
−0.122 |
−0.096 |
−0.058 |
0.023 |
0.191 |
|
Upper gonial angle (Ar-Go-N, °) |
−0.933 |
−0.102 |
0.082 |
0.045 |
−0.048 |
−0.078 |
−0.238 |
|
FH-PP (°) |
−0.926 |
−0.078 |
0.105 |
0.088 |
−0.039 |
0.133 |
−0.136 |
|
S-Ar (mm)*
|
−0.921 |
0.030 |
−0.149 |
0.084 |
0.138 |
−0.097 |
0.155 |
|
Cranial base angle (N-S-Ba, °) |
0.898 |
0.062 |
−0.153 |
−0.115 |
−0.060 |
0.076 |
0.198 |
|
Ar-Go (mm)*
|
−0.895 |
0.125 |
0.139 |
0.000 |
0.129 |
−0.062 |
0.154 |
|
CD-Gn (mm)*
|
−0.835 |
0.035 |
−0.012 |
−0.055 |
0.024 |
−0.077 |
0.454 |
|
Posterior facial height (PFH, S-Go, mm)*
|
−0.831 |
0.172 |
−0.004 |
0.025 |
0.249 |
−0.052 |
0.347 |
|
IMPA (°) |
0.828 |
0.203 |
−0.074 |
0.070 |
−0.006 |
−0.245 |
0.203 |
|
Ar-N (mm)*
|
0.755 |
0.073 |
−0.301 |
−0.095 |
0.016 |
0.139 |
0.489 |
|
FMA (°) |
0.729 |
−0.580 |
0.224 |
0.205 |
0.023 |
0.042 |
−0.032 |
|
Pog-N perpendicular (mm) |
−0.032 |
−0.913 |
−0.036 |
0.221 |
−0.096 |
0.045 |
−0.002 |
|
Facial angle (FH-NPog, °)*
|
−0.009 |
0.866 |
0.042 |
−0.200 |
0.344 |
0.002 |
0.092 |
|
PP-MP (°)*
|
−0.090 |
−0.706 |
0.329 |
0.262 |
−0.097 |
−0.326 |
−0.268 |
|
A-N perpendicular (mm) |
−0.041 |
0.671 |
0.130 |
0.532 |
0.396 |
0.008 |
0.042 |
|
U1-PP (°) |
−0.068 |
0.046 |
0.930 |
−0.029 |
0.231 |
−0.076 |
0.121 |
|
U1-FH (°) |
0.194 |
0.076 |
0.871 |
−0.075 |
0.202 |
−0.317 |
0.118 |
|
U1-OP (°) |
0.526 |
0.227 |
−0.765 |
−0.192 |
−0.010 |
−0.131 |
0.088 |
|
Interincisal angle (°) |
0.509 |
0.209 |
−0.608 |
−0.231 |
0.034 |
0.467 |
0.116 |
|
ANB (°)*
|
−0.064 |
−0.162 |
−0.023 |
0.957 |
0.055 |
−0.067 |
−0.003 |
|
Facial convexity angle (NA-Pog, °) |
0.050 |
0.255 |
−0.061 |
−0.944 |
0.011 |
−0.034 |
0.096 |
|
SNA (°) |
0.009 |
0.257 |
0.156 |
0.253 |
0.892 |
−0.032 |
0.142 |
|
SNB (°) |
0.036 |
0.328 |
0.168 |
−0.138 |
0.885 |
−0.005 |
0.146 |
|
N-ANS/ANS-Me |
0.348 |
0.378 |
−0.361 |
−0.032 |
−0.551 |
0.404 |
0.086 |
|
L1-OP (°) |
0.379 |
0.046 |
−0.205 |
−0.210 |
0.100 |
0.784 |
0.034 |
|
FH-OP (°) |
−0.359 |
−0.379 |
−0.052 |
0.330 |
−0.187 |
0.658 |
−0.150 |
|
OP-MP (°) |
−0.034 |
−0.492 |
0.164 |
−0.062 |
0.188 |
−0.621 |
0.135 |
|
CD-Gn (mm) |
0.621 |
−0.068 |
0.210 |
0.042 |
0.108 |
0.055 |
0.628 |
Mandibular body length/anterior cranial base
(Go-Me/S-N) |
0.104 |
0.261 |
0.135 |
−0.127 |
0.310 |
−0.178 |
0.594 |

Go to :

DISCUSSION
This twin study was the first to compare the heritability of craniofacial skeletal and dental characteristics between twin pairs with skeletal Class I and Class II malocclusions. In previous studies, craniofacial skeletal and dental characteristics were analyzed without considering the differences in the AP skeletal growth patterns.
8,12,14,19
In terms of the AP characteristics, the finding that the heritability values for SNB and facial angle were high in both the Class I and Class II groups (SNB, 0.86 and 0.89, respectively; facial angle, 0.74 and 0.80, respectively;
Table 4) was similar to that of previous studies,
18,19 which reported that SNB was under a strong genetic influence.
In terms of the vertical characteristics, a greater number of cephalometric variables showed high A values for the AP characteristics than for the vertical characteristics in the Class I group (six variables: SNA, SNB, facial convexity angle, facial angle, Pog-N perpendicular, and mandibular body length to anterior cranial base vs. two variables: FH-PP and PP-MP;
Table 4), which was in accordance with the findings of Sidlauskas et al.
19 However, other studies suggested that the vertical variables showed higher heritability than did the AP variables.
8,13 This difference might have originated because of the inclusion of twin samples with different ages or ethnic backgrounds.
In terms of the dental characteristics, the heritability values for the inclination of the maxillary incisors and mandibular incisors as well as the interincisal angle were higher in the Class I group than in the Class II group (U1-FH, 0.82 vs. 0.56; U1-PP, 0.73 vs. 0.59; IMPA, 0.87 vs. 0.58; interincisal angle, 0.75 vs. 0.12;
Table 4). In contrast, the angle between the occlusal plane and the maxillary or mandibular incisors exhibited low-to-moderate heritability in both the Class I and Class II groups (U1-OP, 0.20 and 0.00; L1-OP, 0.21 and 0.43;
Table 4). These findings indicated the need to consider the differences in the pattern of dental compensation of the maxillary and mandibular incisors between patients with Class I and Class II malocclusions.
In terms of the mandibular characteristics, the finding that mandibular body length (Go-Me) had a high A value in the Class I group (0.75;
Table 4) was similar to the results of previous studies.
13,15 However, the Class II group showed a different pattern. (1) The A value of Go-Me was moderate in the Class II group (0.55;
Table 4), and (2) the heritability values for effective mandibular length and ramus height were higher in the Class II group than in the Class I group (CD-Gn, 0.74 vs. 0.00; Ar-Go, 0.81 vs. 0.00;
Table 4). These findings implied that patients with skeletal Class I and Class II malocclusions might have different genetic influences on the size and shape of the mandible. For example, a strong genetic influence was observed on the mandibular body length in patients with skeletal Class I malocclusion and on the overall shape of the mandible and the ramus height in patients with skeletal Class II malocclusion.
In terms of the cranial base characteristics, the saddle angle showed a moderate A value and a low C value in the Class I group (0.68 and 0.00, respectively;
Table 4) and a low A value and a moderate C value in the Class II group (0.00 and 0.68, respectively;
Table 4), which were similar to the results of previous studies.
14,17 Since those studies included younger twins who had not completed growth,
14,17 the heritability estimates of the saddle angle might be low-to-moderate in both younger and adult twins with Class I and Class II malocclusions.
In the present study, PCA extracted eight and seven components with 88.3% and 91.0% cumulative explanation in the Class I and Class II groups, respectively (
Tables 5 and
6). However, previous twin studies reported lower explanation powers than did this study (range: 81.0–83.0%; number of components: 5–9).
8,16,19 The differences between the present study and previous studies might be due to the variations in study designs and statistical criteria used for determining the principal components.
21,22
In summary, the results of this study demonstrated several differences in the genetic heritability of skeletodental characteristics between subjects with Class I and Class II malocclusions. Subjects with Class I malocclusion exhibited strong genetic influences on the facial AP characteristics, mandibular body length, inclination of the maxillary and mandibular incisors, and cranial base angle, whereas those with Class II malocclusion revealed strong genetic influences on the vertical facial height, ramus height, effective mandibular length, and cranial base length (
Table 4). Therefore, the response to orthodontic treatment and/or growth modification treatment in patients with skeletal Class II malocclusion might be different from that of patients with of Class I malocclusion.
Although this study reported the differences in the heritability of skeletodental characteristics between subjects with skeletal Class I and Class II malocclusions, it has several limitations and provides some considerations for future studies. (1) It is necessary to perform a prospective longitudinal study for investigating the growth patterns in twins from childhood, to young adulthood, to middle-age; (2) The number of twins included should be increased to investigate the diverse combinations between the AP (Class I, II, and III relationships) and vertical aspects (normo-, hyper-, and hypo-divergent patterns), (3) Performing more sophisticated statistical analyses and using machine learning for growth prediction would also be helpful, and (4) three-dimensional analysis using low-density cone beam computed tomography images would be necessary to explore the characteristics of transverse growth patterns.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download