Introduction
Laboratory Diagnosis and Surveillance
Recommendations
○ Recommendation 1: The capacity of performing COVID-19 molecular tests and emergency diagnostic tests should be enhanced.
○ Recommendation 2: Strategies should be established for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) variant and antibody testing.
○ Recommendation 3: The capacity of long-term diagnostic testing for infectious diseases should be enhanced by investing in diagnostic testing staff and the necessary infrastructure.
○ Recommendation 4: Research should be continued, and novel methods should be validated for diagnosing infectious diseases.
1. Current national status on COVID-19 diagnostic testing
1) Update on confirmed COVID-19 cases
2) COVID-19 diagnostic testing process implementation in Korea
- January 27, 2020: Initiation of an emergency use authorization system for COVID-19 testing.
- February 4, 2020: Final approval of COVID-19 test kits for the first time.
- February 7, 2020: Initiation of COVID-19 testing in hospitals and commercial laboratories.
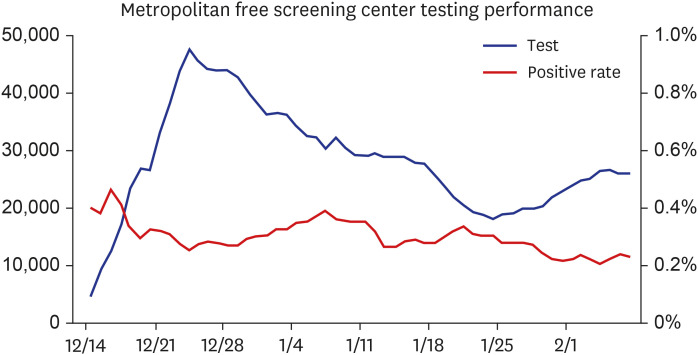
- April 1, 2021: Completion of a total of 15 million COVID-19 molecular tests for 26 million people at 146 clinical laboratories (Fig. 1).
3) Characteristics and types of COVID-19 testing methods in Korea
(1) Molecular test
(2) Antigen test
(3) Antibody test
4) Background of the success of COVID-19 diagnostic testing in Korea
(1) Excellent human resources and policies
(2) Active molecular testing market
(3) Fee-for-service
(4) Emergency use approval system for in vitro diagnostics
(5) Collaboration between laboratory medicine experts and disease control authorities
2. Short-term pending issues and plans related to COVID-19 diagnostic testing
1) Effective use of various diagnostic testing methods
(1) Discontinuation of the antigen test
(2) Careful use of saliva specimens
(3) Active use of pooled assays
2) Increased capacity to perform diagnostic tests
(1) Expansion of free screening centers
(2) Expansion of staff for diagnostic testing and specimen collection
(3) Increase in the capacity of rapid molecular testing
Table 1
Status of diagnostic testing reagent approval for COVID-19 rapid molecular testing in Korea (as of July 26, 2021)
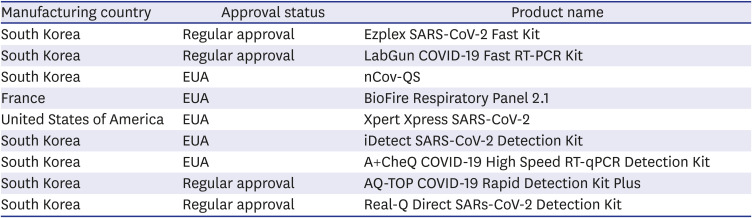
(4) Implementation of automated testing devices
3. Mid-to-long-term vision and plan for COVID-19 diagnostic testing
1) Foreign introduction of viral variants and the corresponding plan
(1) Continuous reporting of novel variants
Table 2
Main variants of concern and their characteristics (source: CDC.gov)
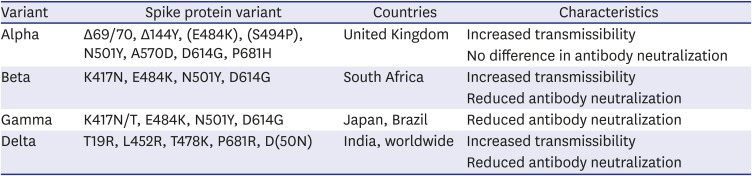
(2) Screening test strategies for preventing the introduction of variants
(3) Surveillance using whole-genome sequencing (WGS)
2) Strategies for evaluating COVID-19 vaccine efficacy using the antibody test
3) Strategies for the diagnosis of respiratory viruses and COVID-19 in the future
4) Development of mid-to-long-term diagnostic testing methods and strategies for management
(1) Active participation of laboratory medicine specialists in policy-making
(2) Expansion of diagnostic testing capacity: staff, biosafety facilities, and laboratories
(3) Development of new infectious disease testing methods and long-term evaluation
4. Summary
Treatments and Vaccine Research
Recommendations
○ Recommendation 5: The transmission characteristics of SARS-CoV-2 mutants and the efficacy of vaccines should be studied.
○ Recommendation 6: Supplies of and systems for approved treatments should be established.
○ Recommendation 7: Preparations should be ongoing for a prolonged COVID-19 outbreak, and efficient treatments should be developed for patients with mild symptoms.
○ Recommendation 8: Follow-up studies on the duration of the effects of vaccines and on the long-term safety of vaccines, in addition to blocking community transmission, are needed.
○ Recommendation 9: Support for domestic vaccine and therapeutic development should be continued, and information on development and clinical applications should be disclosed.
○ Recommendation 10: The final vaccine to be used in Korea should be selected after considering the efficacy and safety of the vaccine.
○ Recommendation 11: After thorough preparation and enhancement of public confidence, the vaccination rate should be increased to achieve herd immunity.
1. COVID-19 causative virus
1) Status
2) Problems and recommendations
2. Development of therapeutics
1) Status
2) Problems and recommendations
3. Vaccine development
1) Status
2) Current status of approved COVID-19 vaccines in other countries
Table 3
Severe acute respiratory syndrome-coronavirus-2 vaccines approved for emergency use by country
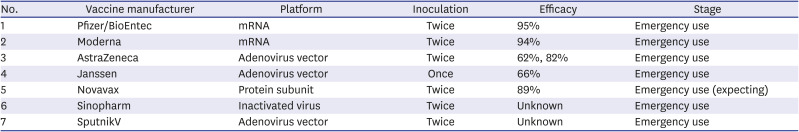
3) Current status of COVID-19 vaccine approvals in Korea
Table 4
Current status of domestic coronavirus disease 2019 vaccine development
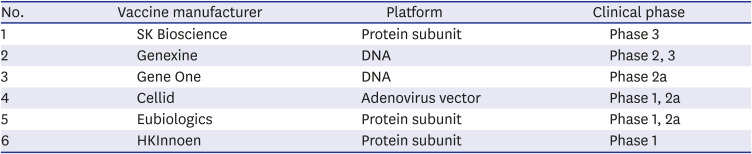
4) Further problems and recommendations
Critical Care of COVID-19 Patients
1. Critically ill cases occurred during the last 1-year pandemic in Korea
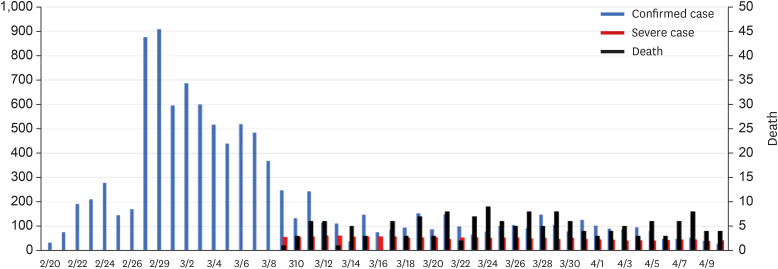 | Fig. 3Status of confirmed cases, critically ill cases, and deaths during the first coronavirus disease 2019 pandemic in Korea. There is no information on critically ill patients during the early stages of the COVID-19 outbreak in the government data. The Korean Society of Critical Care Medicine collected critical care data from March 9, focusing on training hospitals specializing in critical care medicine. In this graph, critical patients are those requiring mechanical ventilation. |
 | Fig. 4Status of confirmed cases, critically ill cases, and deaths during the second and third coronavirus disease 2019 pandemics. In this graph, critical patients are those requiring high-flow oxygen therapy, mechanical ventilation, extracorporeal membrane oxygenation, continuous renal replacement therapy, etc. |
2. The impact of mutant viruses on the increase in critical cases in the UK
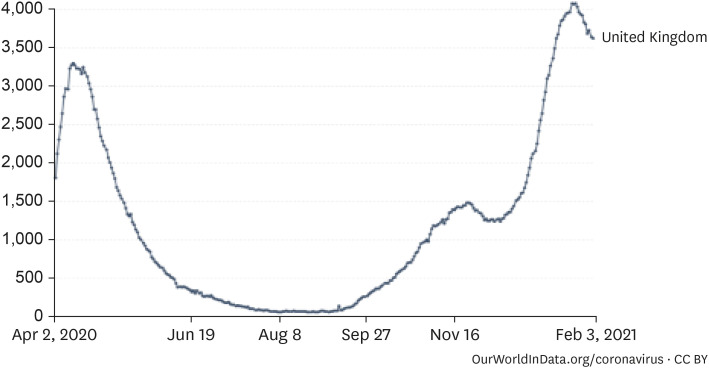 | Fig. 5Coronavirus disease 2019 inpatient trends in the UK (7-day average).27Source: European CDC for EU countries, government sources for other countries.
|
3. Strategies for intensive care
1) Status of treatment for critically ill COVID-19 patients
Table 5
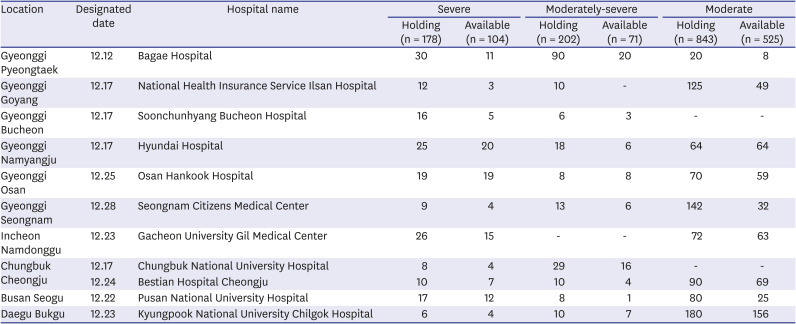
Status of hospitals designated to infectious diseases (March 13, 2020)

Table 7
Status of functioning of hospital beds dedicated to coronavirus disease 2019 (press release on January 21, 2021)
2) COVID-19 critical care status in the USA
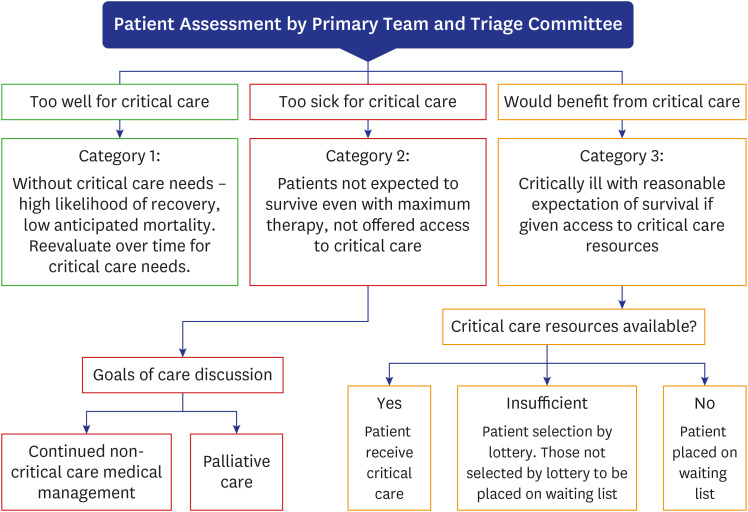
4. Ethical considerations
5. Recommendations
Mental Health of COVID-19 Patients
Recommendations
○ Recommendation 15: Psychiatric issues related to COVID-19 are diverse, and in-depth domestic research is needed.
○ Recommendation 16: It is necessary to identify problems with the use of programs to heal psychiatric problems caused by COVID-19 and to develop programs that can supplement the same.
○ Recommendation 17: In order to solve psychiatric issues, systematic recommendations or suggestions are needed to prevent psychological instability as a result of COVID-19.
1. Psychiatric disorders related to COVID-19
1) Current situation
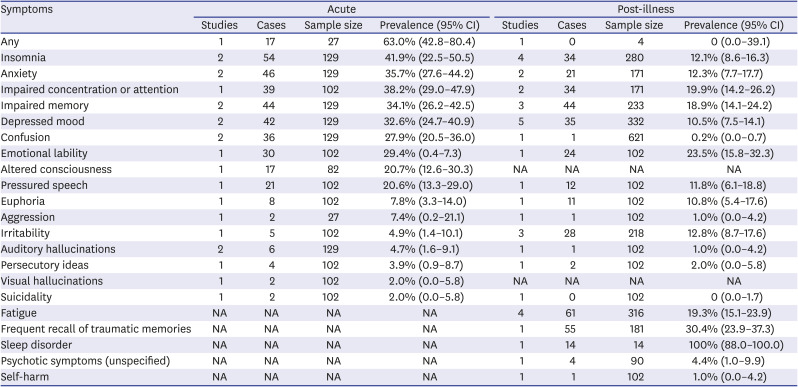
(1) Psychiatric symptoms in COVID-19 patients
Table 8
Prevalence of psychiatric symptoms in infected persons33
(2) Impact on therapists
 | Fig. 8Psychiatric symptoms found among therapists.34PTSD = posttraumatic stress disorder.
|
(3) Psychiatric effects on the general public
(4) Psychiatric conditions found in high-risk groups
2) Domestic research
(1) Research on the general population
(2) Research on the mentally ill population
 | Fig. 9Underlying psychiatric disorders, use of antipsychotics, and SARS-CoV-2.43OR = odd ratio, CI = confidence interval, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, COVID-19 = coronavirus diseases 2019.
|
2. Corona blue prevention and treatment measures
1) Review of the psychological prevention protocol of the trauma center of the National Center for Mental Health
(1) Tips for good mental health (Fig. 10)
(2) The psychological support operating system
2) Review of response plans of Seoul and other local governments
3) Suggesting the most appropriate psychological prevention measures for the future
Policy and Legislation
Recommendations
○ Recommendation 18: The government should set and manage patient outbreak suppression targets by region and period based on the existing three quarantine measures consisting of testing, tracking, and quarantining individuals with suspected infection.
○ Recommendation 19: People should strictly comply with three actions, i.e., wearing a mask, washing hands, and following coughing etiquettes, to prevent the spread of COVID-19.
○ Recommendation 20: People should maintain distance between each other and minimize the time spent in a 3C environment (i.e., closed space, close contact, and crowded place) to reduce the possibility of transmitting COVID-19.
○ Recommendation 21: The government should evaluate the risk level in various environments based on evidence and continuously improve social distancing guidelines accordingly.
○ Recommendation 22: The government should strengthen the facilitating factors of nonpharmacological interventions, make efforts to remove obstacles, and induce desirable behaviors in people through active risk communication.
○ Recommendation 23: The government should strengthen the capabilities of the healthcare system, including personnel, facilities, and equipment, to provide affordable treatment to COVID-19 patients.
○ Recommendation 24: The government should embark on the long-term enhancement of public health capacity, strengthening of public–private cooperation, and development of a new future healthcare delivery model.
○ Recommendation 25: As the variants that have recently spread globally have affected the speed of transmission, disease severity, and vaccine effectiveness, the surveillance network should be expanded, rapid contact tracing and isolation should be performed, and rapid effective vaccine roll-out should be prioritized in order to prevent the variants from entering Korea and causing a new epidemic.
1. Current situation and progress
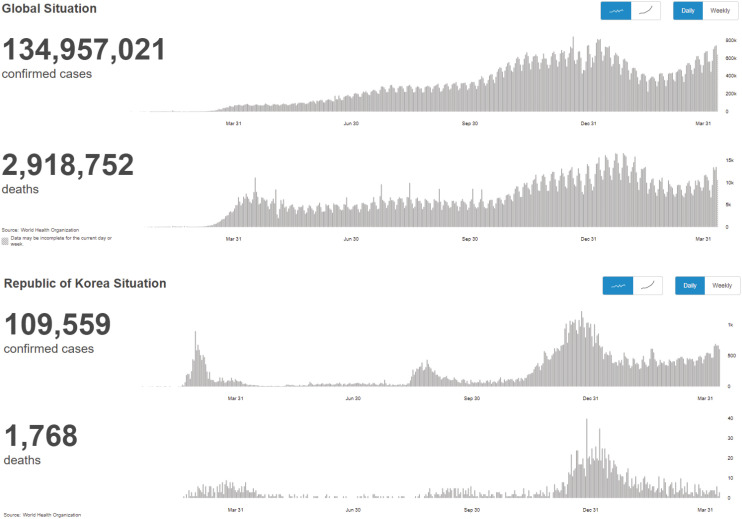
1) Status of occurrence44
2) Republic of Korea's COVID-19 response
(1) First epidemic wave
(2) Second epidemic wave
(3) Third epidemic wave
3) Main activities of the NAMOK
Table 9
The status of online forum in response to COVID-19 (as of March 2021)





 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download