1. Sooriakumaran P, Srivastava A, Shariat SF, Stricker PD, Ahlering T, Eden CG, et al. A multinational, multi-institutional study comparing positive surgical margin rates among 22393 open, laparoscopic, and robot-assisted radical prostatectomy patients. Eur Urol. 2014; 66(3):450–456. PMID:
24290695.
2. Preisser F, Mazzone E, Knipper S, Nazzani S, Bandini M, Shariat SF, et al. Rates of positive surgical margins and their effect on cancer-specific mortality at radical prostatectomy for patients with clinically localized prostate cancer. Clin Genitourin Cancer. 2019; 17(1):e130–e139. PMID:
30366880.

3. Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998; 160(2):299–315. PMID:
9679867.

4. Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996; 23(4):651–663. PMID:
8948418.

5. Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 5: surgical margins. Mod Pathol. 2011; 24(1):48–57. PMID:
20729812.

6. Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, Yuan J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018; 16(1):124. PMID:
29970100.

7. Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol. 2009; 55(1):87–99. PMID:
18838211.

8. Dev HS, Wiklund P, Patel V, Parashar D, Palmer K, Nyberg T, et al. Surgical margin length and location affect recurrence rates after robotic prostatectomy. Urol Oncol. 2015; 33(3):109.e7–109.13.

9. Fontenot PA, Mansour AM. Reporting positive surgical margins after radical prostatectomy: time for standardization. BJU Int. 2013; 111(8):E290–E299. PMID:
23489974.

10. Wu S, Lin SX, Wirth GJ, Lu M, Lu J, Subtelny AO, et al. Impact of multifocality and multilocation of positive surgical margin after radical prostatectomy on predicting oncological outcome. Clin Genitourin Cancer. 2019; 17(1):e44–e52. PMID:
30287224.

11. Sooriakumaran P, Dev HS, Skarecky D, Ahlering T. The importance of surgical margins in prostate cancer. J Surg Oncol. 2016; 113(3):310–315. PMID:
27004601.

12. Shikanov S, Marchetti P, Desai V, Razmaria A, Antic T, Al-Ahmadie H, et al. Short (≤ 1 mm) positive surgical margin and risk of biochemical recurrence after radical prostatectomy. BJU Int. 2013; 111(4):559–563. PMID:
22759270.
13. Lee S, Kim KB, Jo JK, Ho JN, Oh JJ, Jeong SJ, et al. Prognostic value of focal positive surgical margins after radical prostatectomy. Clin Genitourin Cancer. 2016; 14(4):e313–e319. PMID:
27130538.

14. Cao D, Humphrey PA, Gao F, Tao Y, Kibel AS. Ability of linear length of positive margin in radical prostatectomy specimens to predict biochemical recurrence. Urology. 2011; 77(6):1409–1414. PMID:
21256540.

15. Kates M, Sopko NA, Han M, Partin AW, Epstein JI. Importance of reporting the Gleason score at the positive surgical margin site: analysis of 4,082 consecutive radical prostatectomy cases. J Urol. 2016; 195(2):337–342. PMID:
26264998.

16. Savdie R, Horvath LG, Benito RP, Rasiah KK, Haynes AM, Chatfield M, et al. High Gleason grade carcinoma at a positive surgical margin predicts biochemical failure after radical prostatectomy and may guide adjuvant radiotherapy. BJU Int. 2012; 109(12):1794–1800. PMID:
21992536.

17. Chalfin HJ, Dinizo M, Trock BJ, Feng Z, Partin AW, Walsh PC, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012; 110(11):1684–1689. PMID:
22788795.

18. Stephenson AJ, Eggener SE, Hernandez AV, Klein EA, Kattan MW, Wood DP Jr, et al. Do margins matter? The influence of positive surgical margins on prostate cancer-specific mortality. Eur Urol. 2014; 65(4):675–680. PMID:
24035631.

19. Mithal P, Howard LE, Aronson WJ, Terris MK, Cooperberg MR, Kane CJ, et al. Positive surgical margins in radical prostatectomy patients do not predict long-term oncological outcomes: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) cohort. BJU Int. 2016; 117(2):244–248. PMID:
26010160.

21. Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, Joensuu T, et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol. 2019; 76(5):586–595. PMID:
31375279.

22. Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012; 380(9858):2018–2027. PMID:
23084481.

23. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009; 181(3):956–962. PMID:
19167731.

24. Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014; 66(2):243–250. PMID:
24680359.

25. Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020; 396(10260):1422–1431. PMID:
33002431.

26. Shih TH, Fan X. Comparing response rates in e-mail and paper surveys: a meta-analysis. Educ Res Rev. 2009; 4(1):26–40.

27. Hox JJ, De Leeuw ED. A comparison of nonresponse in mail, telephone, and face-to-face surveys. Qual Quant. 1994; 28(4):329–344.

28. Meade AW, Craig SB. Identifying careless responses in survey data. Psychol Methods. 2012; 17(3):437–455. PMID:
22506584.

29. Ong AD, Weiss DJ. The impact of anonymity on responses to sensitive questions 1. J Appl Soc Psychol. 2000; 30(8):1691–1708.
30. Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002; 167(1):112–116. PMID:
11743286.

31. Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002; 90(6):561–566. PMID:
12230618.

32. Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003; 170(3):791–794. PMID:
12913699.

33. Akitake N, Shiota M, Obata H, Takeuchi A, Kashiwagi E, Imada K, et al. Neoadjuvant androgen-deprivation therapy with radical prostatectomy for prostate cancer in association with age and serum testosterone. Prostate Int. 2018; 6(3):104–109. PMID:
30140660.

34. McClintock TR, von Landenberg N, Cole AP, Lipsitz SR, Gild P, Sun M, et al. Neoadjuvant androgen deprivation therapy prior to radical prostatectomy: recent trends in utilization and association with postoperative surgical margin status. Ann Surg Oncol. 2019; 26(1):297–305. PMID:
30430324.

35. Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012; 62(1):1–15. PMID:
22405509.

36. Basiri A, de la Rosette JJ, Tabatabaei S, Woo HH, Laguna MP, Shemshaki H. Comparison of retropubic, laparoscopic and robotic radical prostatectomy: who is the winner? World J Urol. 2018; 36(4):609–621. PMID:
29362896.

37. Guazzoni G, Cestari A, Naspro R, Riva M, Centemero A, Zanoni M, et al. Intra- and peri-operative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol. 2006; 50(1):98–104. PMID:
16563608.

38. Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016; 388(10049):1057–1066. PMID:
27474375.

39. D'Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999; 17(1):168–172. PMID:
10458230.
40. Lavery HJ, Nabizada-Pace F, Carlucci JR, Brajtbord JS, Samadi DB. Nerve-sparing robotic prostatectomy in preoperatively high-risk patients is safe and efficacious. Urol Oncol. 2012; 30(1):26–32. PMID:
20189844.

41. Kumar A, Samavedi S, Bates AS, Mouraviev V, Coelho RF, Rocco B, et al. Safety of selective nerve sparing in high risk prostate cancer during robot-assisted radical prostatectomy. J Robot Surg. 2017; 11(2):129–138. PMID:
27435701.

42. Takahara K, Sumitomo M, Fukaya K, Jyoudai T, Nishino M, Hikichi M, et al. Clinical and oncological outcomes of robot-assisted radical prostatectomy with nerve sparing vs. non-nerve sparing for high-risk prostate cancer cases. Oncol Lett. 2019; 18(4):3896–3902. PMID:
31579411.

43. Greco F, Hoda MR, Wagner S, Reichelt O, Inferrera A, Magno C, et al. Bilateral vs unilateral laparoscopic intrafascial nerve-sparing radical prostatectomy: evaluation of surgical and functional outcomes in 457 patients. BJU Int. 2011; 108(4):583–587. PMID:
21091973.

44. Kim M, Park M, Pak S, Choi SK, Shim M, Song C, et al. Integrity of the urethral sphincter complex, nerve-sparing, and long-term continence status after robotic-assisted radical prostatectomy. Eur Urol Focus. 2019; 5(5):823–830. PMID:
29759661.

45. Vickers A, Bianco F, Cronin A, Eastham J, Klein E, Kattan M, et al. The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010; 183(4):1360–1365. PMID:
20171687.

46. Secin FP, Savage C, Abbou C, de La Taille A, Salomon L, Rassweiler J, et al. The learning curve for laparoscopic radical prostatectomy: an international multicenter study. J Urol. 2010; 184(6):2291–2296. PMID:
20952022.

47. Bravi CA, Tin A, Vertosick E, Mazzone E, Martini A, Dell'Oglio P, et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol. 2019; 202(1):108–113. PMID:
30747873.

48. Cangiano TG, Litwin MS, Naitoh J, Dorey F, deKernion JB. Intraoperative frozen section monitoring of nerve sparing radical retropubic prostatectomy. J Urol. 1999; 162(3 Pt 1):655–658. PMID:
10458335.
49. Tsuboi T, Ohori M, Kuroiwa K, Reuter VE, Kattan MW, Eastham JA, et al. Is intraoperative frozen section analysis an efficient way to reduce positive surgical margins? Urology. 2005; 66(6):1287–1291. PMID:
16360458.

50. Lavery HJ, Xiao GQ, Nabizada-Pace F, Mikulasovich M, Unger P, Samadi DB. ‘Mohs surgery of the prostate’: the utility of in situ frozen section analysis during robotic prostatectomy. BJU Int. 2011; 107(6):975–979. PMID:
20880130.

51. Heinrich E, Schön G, Schiefelbein F, Michel MS, Trojan L. Clinical impact of intraoperative frozen sections during nerve-sparing radical prostatectomy. World J Urol. 2010; 28(6):709–713. PMID:
20358209.

52. Kakiuchi Y, Choy B, Gordetsky J, Izumi K, Wu G, Rashid H, et al. Role of frozen section analysis of surgical margins during robot-assisted laparoscopic radical prostatectomy: a 2608-case experience. Hum Pathol. 2013; 44(8):1556–1562. PMID:
23561622.

53. Schlomm T, Tennstedt P, Huxhold C, Steuber T, Salomon G, Michl U, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012; 62(2):333–340. PMID:
22591631.
54. Mirmilstein G, Rai BP, Gbolahan O, Srirangam V, Narula A, Agarwal S, et al. The neurovascular structure-adjacent frozen-section examination (NeuroSAFE) approach to nerve sparing in robot-assisted laparoscopic radical prostatectomy in a British setting - a prospective observational comparative study. BJU Int. 2018; 121(6):854–862. PMID:
29124889.
55. Preisser F, Theissen L, Wild P, Bartelt K, Kluth L, Köllermann J, et al. Implementation of intraoperative frozen section during radical prostatectomy: short-term results from a German tertiary-care center. Eur Urol Focus. 2021; 7(1):95–101. PMID:
30905598.

56. Öbek C, Saglican Y, Ince U, Argun OB, Tuna MB, Doganca T, et al. Intra-surgical total and re-constructible pathological prostate examination for safer margins and nerve preservation (Istanbul preserve). Ann Diagn Pathol. 2018; 33:35–39. PMID:
29566945.

57. Abdollah F, Sun M, Suardi N, Gallina A, Capitanio U, Bianchi M, et al. Presence of positive surgical margin in patients with organ-confined prostate cancer equals to extracapsular extension negative surgical margin. A plea for TNM staging system reclassification. Urol Oncol. 2013; 31(8):1497–1503. PMID:
22591746.

58. Hashimoto T, Yoshioka K, Horiguchi Y, Inoue R, Yoshio O, Nakashima J, et al. Clinical effect of a positive surgical margin without extraprostatic extension after robot-assisted radical prostatectomy. Urol Oncol. 2015; 33(12):503.e1–503.e6.

59. Lee IJ, Oh JJ, Kim TJ, Song BD, Lee S, Hong SK, et al. Clinical significance of positive surgical margin after radical prostatectomy according to pathological stage. Korean J Urol Oncol. 2016; 14(3):159–164.

60. Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014; 65(2):303–313. PMID:
23932439.

61. Yanai Y, Matsumoto K, Kosaka T, Takeda T, Tanaka N, Morita S, et al. External validation of the “optimal PSA follow-up schedule after radical prostatectomy” in a new cohort. Int J Clin Oncol. 2020; 25(7):1393–1397. PMID:
32285217.

62. Lightner DJ, Lange PH, Reddy PK, Moore L. Prostate specific antigen and local recurrence after radical prostatectomy. J Urol. 1990; 144(4):921–926. PMID:
1697917.

63. De Visschere PJ, Standaert C, Fütterer JJ, Villeirs GM, Panebianco V, Walz J, et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol. 2019; 2(1):47–76. PMID:
30929846.

64. Robertson NL, Sala E, Benz M, Landa J, Scardino P, Scher HI, et al. Combined whole body and multiparametric prostate magnetic resonance imaging as a 1-step approach to the simultaneous assessment of local recurrence and metastatic disease after radical prostatectomy. J Urol. 2017; 198(1):65–70. PMID:
28216327.

65. Pisansky TM, Thompson IM, Valicenti RK, D'Amico AV, Selvarajah S. Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA guideline amendment 2018–2019. J Urol. 2019; 202(3):533–538. PMID:
31042111.

67. Balakrishnan AS, Zhao S, Cowan JE, Broering JM, Cooperberg MR, Carroll PR. Trends and predictors of adjuvant therapy for adverse features following radical prostatectomy: an analysis from cancer of the prostate strategic urologic research endeavor. Urology. 2019; 131:157–165. PMID:
31150694.

68. Shaikh MP, Alite F, Wu MJ, Solanki AA, Harkenrider MM. Adjuvant radiotherapy versus wait-and-see strategy for pathologic T3 or margin-positive prostate cancer: a meta-analysis. Am J Clin Oncol. 2018; 41(8):730–738. PMID:
28225445.






 PDF
PDF Citation
Citation Print
Print



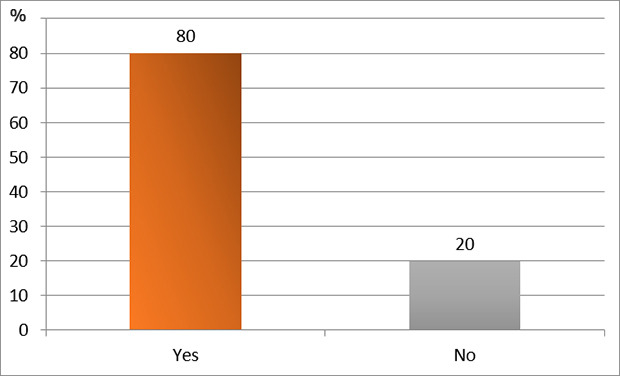
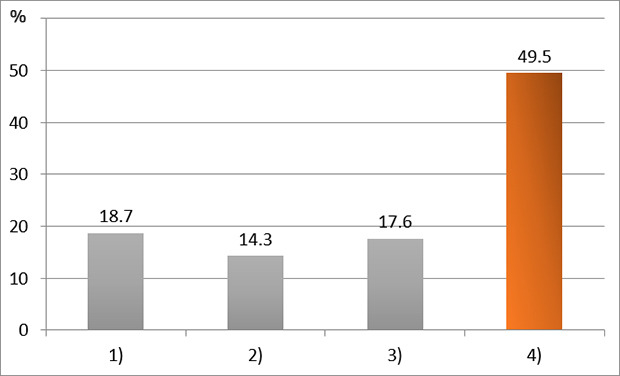
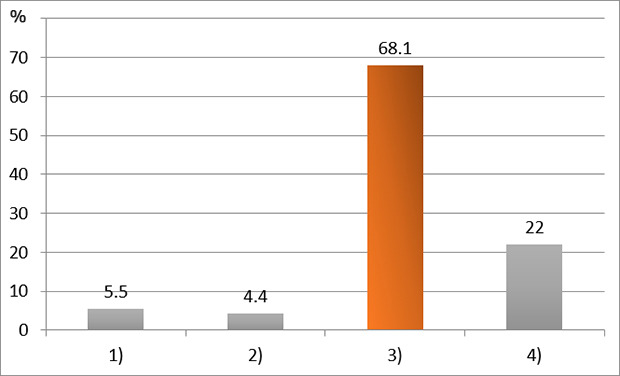
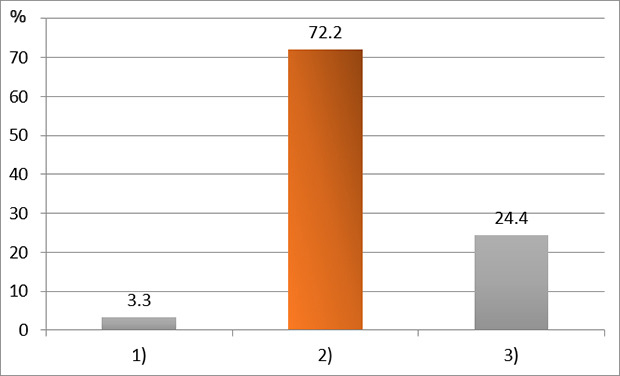
 XML Download
XML Download