Abstract
The present article demonstrates an unusual case of type 1 autoimmune pancreatitis (AIP), focusing on the cause of deterioration in glycemic control and weight loss in a patient with pre-existing type 2 diabetes mellitus (DM). A 67-year-old man was diagnosed with type 2 DM 23 years prior and presented with weight loss of approximately 6 kg over a period of 3 months and hemoglobin A1c (HbA1c) of 9.1%. His carbohydrate antigen 19-9 (CA 19-9) level was elevated to 158.54 U/mL (normal, 0~37 U/mL). Considering these findings, we needed to rule out hidden malignancy. Computed tomography of the abdomen showed diffuse swelling of the pancreas uncinated process. The serum immunoglobulin G (IgG) level was elevated to 2,418 mg/dL (normal, 700~1,600 mg/dL), and IgG4 level was elevated to 1,115.0 mg/dL (normal range, 3.9~86.4 mg/dL). This case highlights that AIP should be considered as a cause of significant weight loss and a deterioration in glycemic control in patients with DM. Furthermore, a pancreatic imaging study should be considered in clinical practice to differentiate pancreatic cancer and AIP.
Go to : 
Autoimmune pancreatitis (AIP), a unique subtype of pancreatitis, is often accompanied by systemic inflammatory disorders. There are two types of AIP depending on the pathologic features: lymphoplasmacytic sclerosing pancreatitis (type 1 AIP) and granulocytic epithelial lesions (type 2 AIP). Type 1 AIP is considered a pancreatic manifestation of immunoglobulin G (IgG) 4-related disease [1]. AIP is common in elderly individuals, and approximately 40~80% of AIP cases are reported to accompany diabetes mellitus (DM) [2–5]. The impact of chronic pancreatic inflammation of AIP can influence glucose metabolism [2–5]. In this report, we present a type 1 AIP case, focusing on the cause of deterioration in glycemic control and weight loss in a patient with pre-existing type 2 DM.
Go to : 
A 67-year-old man presented to the diabetic clinic because of poor glucose control for 2 months despite oral hypoglycemic therapy. He had been diagnosed with type 2 DM 23 years prior, and he presented with dry mouth, weight loss of approximately 6 kg over a period of 3 months, general fatigue, and hemoglobin A1c (HbA1c) of 9.1%. During the previous 2 months, he showed no change in either amount of food intake or physical activity. His past medical history included asthma, benign prostatic hypertrophy, and treated hypertension. A previous trial of gemigliptin (50 mg), metformin (2,000 mg), and glimepiride (4 mg) daily had been effective in lowering his blood glucose level, with an HbA1c value between 6% and 7%. Upon admission to our hospital, a physical examination revealed height 165 cm, weight 58 kg, body mass index 21 kg/m2, body temperature 36.6℃, blood pressure 120/80 mmHg, and a regular heart rate of 70 beats/min. His cardiovascular and lung examination results were normal. A nutritional assessment was performed. He denied using herbal supplements or change in daily food consumption and caloric intake. Laboratory findings revealed a serum glucose of 337 mg/dL without evidence of ketoacidosis; leukocytes, 8,800/μ L; hemoglobin, 13.5 g/dL; platelets, 197,000/μL; aspartate aminotransferase, 20 U/L (normal, 7~38 U/L); alanine aminotransferase, 14 U/L (normal, 4~34 U/L); alkaline phosphatase, 233 IU/L (normal, 104~338 IU/L); total bilirubin, 0.4 mg/dL (normal, 0.3~1.2 mg/dL); amylase, 171 U/L (normal, 40~126 U/L); lipase, 331 U/L (normal, 13~55 U/L); and C-reactive protein, 0.02 mg/dL (normal, 0~0.3 mg/dL). His carbohydrate antigen 19-9 (CA 19-9) level was elevated to 158.54 U/mL (normal, 0~37 U/mL), and prostate specific antigen level was 0.79 ng/mL (normal, 0~4 ng/mL). Other blood chemistry levels revealed blood urea nitrogen 26 mg/dL (normal, 8~20 mg/dL), creatinine 1.3 mg/dL (normal, 0.6~1.2 mg/dL), total protein 7.9 g/dL (normal, 6~8.1 g/dL), serum albumin 4.3 g/dL (normal, 3.5~5.3 g/dL), sodium 137 mEq/L (normal, 135~145 mEq/L), and potassium 4.9 mEq/L (normal, 3.5~5.3 mEq/L). The results of the thyroid function tests were within normal limits. The fasting c-peptide level was 1.30 ng/mL (normal, 0.5~3.5 ng/mL), and anti-glutamic acid decarboxylase antibody level was 0.7 U/mL (normal, <1.0 U/mL). The calculated HOMA-IR (homeostatic model assessment insulin resistance) was 0.42, and HOMA-β was 6.8. Serum protein electrophoresis was normal.
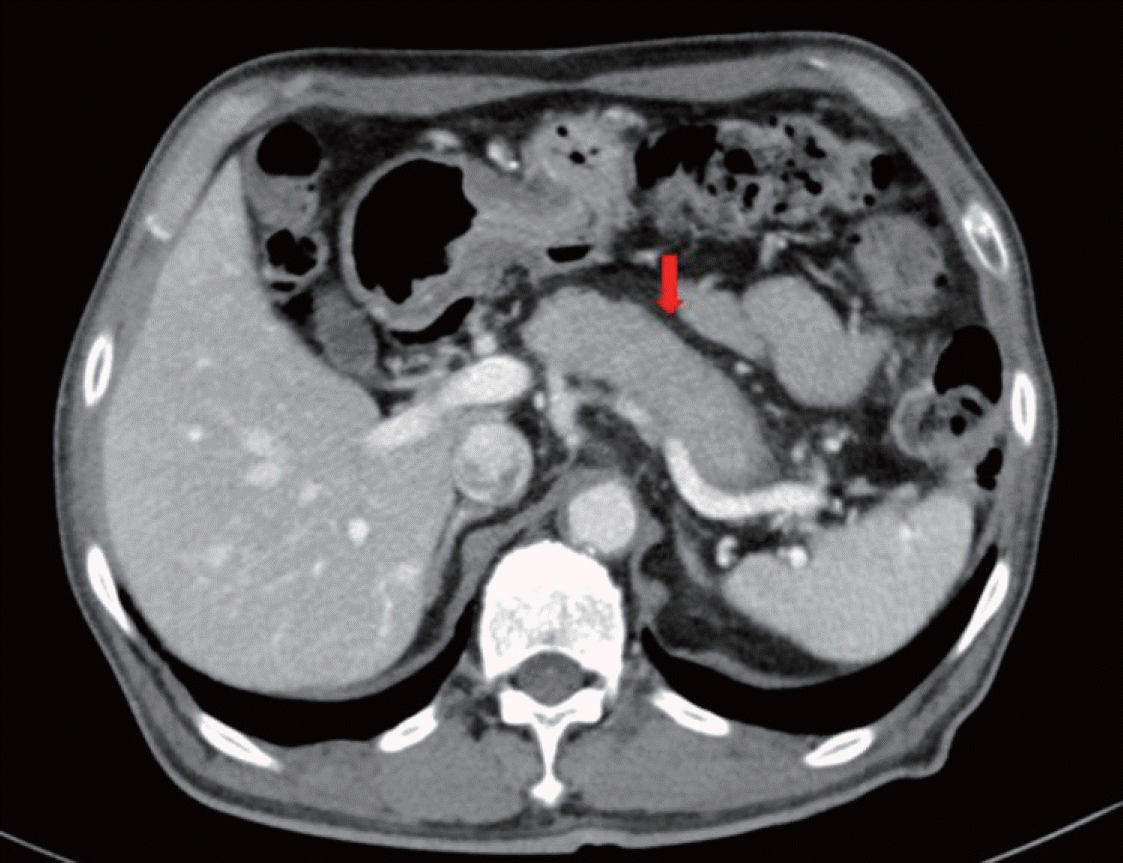
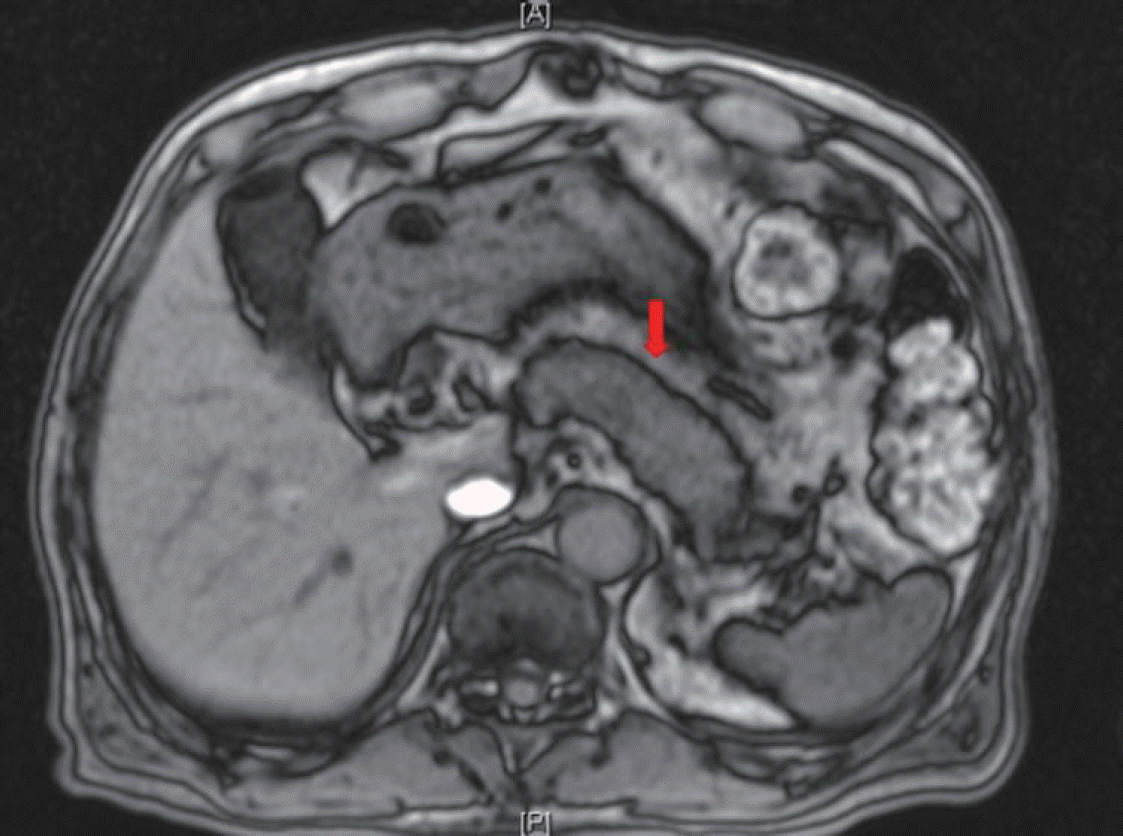
The patient was administered insulin aspart three times and basal insulin degludec daily to optimize his glucose control. Notably, the patient underwent endoscopic evaluation for unintentional weight loss and showed no clinically significant findings. He underwent chest and abdominal computed tomography (CT) to rule out malignancy. Abdominal CT showed diffuse swelling of the pancreas uncinated process (Fig. 1). Magnetic resonance imaging (MRI) was performed to differentiate between diffuse infiltrative pancreatic diseases such as AIP and an iso-attenuating pancreatic tumor. Pancreatic MRI showed diffuse swelling of the pancreas without any definite mass lesion and normal pancreatic duct (Fig. 2). The results were more compatible with AIP than pancreatic tumor.
Therefore, an additional workup was required. There was no recent viral infection, including hepatitis A, hepatitis B, hepatitis C, cytomegalovirus, and Epstein-Barr virus. All tests for anti-nuclear antibodies, rheumatoid factor, anti-mitochondrial antibody, anti-liver kidney microsomal 1 antibody, and anti-smooth muscle antibody were negative. The serum IgG level was elevated to 2,418 mg/dL (normal, 700~1,600 mg/dL), and IgG4 level was elevated to 1,115.0 mg/dL (normal, 3.9~86.4 mg/dL). Endoscopic-ultrasound-guided fine-needle aspiration (EUS-FNA) was not performed because the CT and MRI images showed typical AIP features lacking focal mass-like lesions, and serum IgG4 level was more than 10 times higher than the normal upper limit.
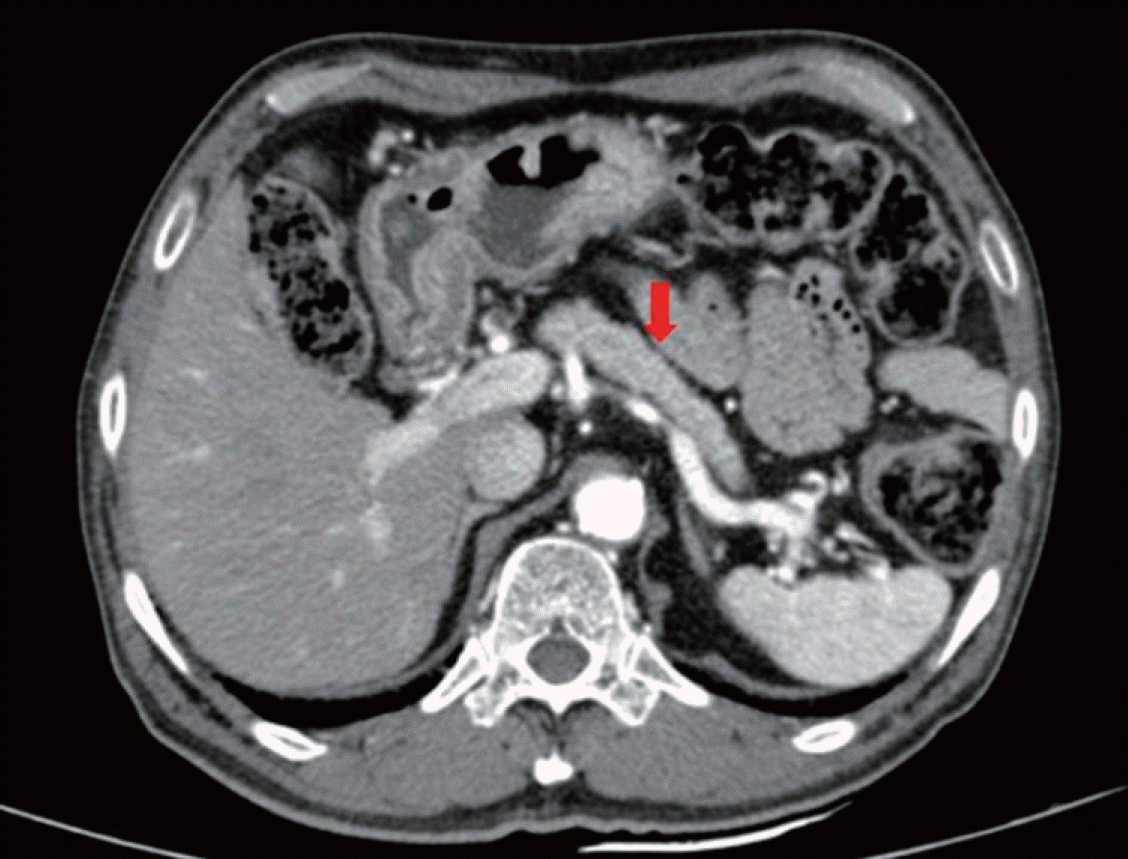
Based on clinical, laboratory, and imaging findings, we diagnosed our patient with type 1 AIP. He was treated with oral prednisolone (40 mg/day) (0.6 mg/kg) for 4 weeks, which was reduced by 5 mg/day every 2 weeks and gradually tapered to a maintenance dose of 2.5 mg/day, according to the international consensus for treatment of AIP without recurrence. Glycemic control worsened transiently after initiation of steroids. After glycemic control was stabilized, the calculated HOMA-IR was 0.25, and HOMA-β was 10.3. Follow-up with contrast-enhanced abdominal CT was performed after 2 months of steroid treatment and showed marked improvement of pancreatic enlargement (Fig. 3). Insulin requirements were expected to decline along with prednisolone tapering and AIP improvement. After 5 months of steroid treatment, his IgG4 level decreased to 532 mg/dL (normal, 3.9~86.4 mg/dL) and his CA 19-9 level normalized to 22.18 U/mL (normal, 0~37 U/mL).
The patient needed a maximum insulin dose of 36 units/day with oral hypoglycemic agents after initiating steroid therapy. However, the dose was decreased to 12 units/day after prednisolone had been tapered to 2.5 mg/day, with an HbA1c level of 8.0%. Follow up with contrast-enhanced abdominal CT at 10 months of steroid treatment showed normalization in pancreas volume and resolution of the peri-pancreatic halo. At this followup, he showed a regain of 5 kg in weight. He is currently doing well with glycemic control on a low dose of prandial insulin, metformin 1 g daily, and glimepiride 4 mg daily. To prevent a relapse of AIP, he has been taking oral prednisolone 2.5 mg daily for 6 months.
Go to : 
AIP is a rare chronic pancreatic disease induced by autoimmune mechanisms, increasingly recognized in the recent years [6]. Jaundice, weight loss, and anorexia are common in AIP patients. Serum CA 19-9 level, which is an important pancreatic adenocarcinoma (PAC) marker, also shows a marked increase in AIP patients. Similar reports of a significant increase of serum CA 19-9 level above the normal range have been detailed in many AIP patients [7]. Cross-sectional images are used for differential diagnosis because the symptoms and laboratory findings are nonspecific, and there are similarities between AIP and pancreatic-biliary malignancies. In this case, pancreatic imaging was performed due to remarkably increased serum CA 19-9 level and worsening of glycemic control associated with weight loss to rule out PAC. In previous reports, approximately 30~40% of AIP patients with DM had a history of diabetic treatment before AIP onset [2,8]. Notably, worsening of glycemic control can lead to AIP.
Diagnosis of AIP usually involves imaging, serology, histology, other organ involvement, and response to steroid [9,10]. Among these, imaging findings such as those of CT/MRI and ERCP (endoscopic retrograde cholangiopancreatography)/MRCP (magnetic resonance cholangiopancreatography) are important. However, diagnosis of diffuse-type AIP can be based on typical CT or MRI findings with other findings (serology, histology, other organ involvement) but without ERCP or MRCP imaging [9,10]. Imaging methods cannot distinguish among AIP subtypes and show diffuse (typical) and focal (segmental) pancreatic involvement. In this case, for diffuse-type AIP, a sausage-shaped pancreas with a capsule-like rim on abdominal CT is a particular and typical feature. Consequently, it is rarely necessary to differentiate diffuse-type AIP from PAC. However, the possibility of simultaneous co-existence of AIP (or IgG4-related pathology) and pancreatic malignancies, such as a small invasive cancer within the lesion of AIP [11], a concomitant pancreatic mixed acinar-ductal adenocarcinoma, and follicular lymphoma accompanied with IgG4-related pathology should be considered [12]. In the international guideline, a persistent pancreatic mass indicates steroid therapy after negative workup for pancreatic malignancies by EUS-FNA, even in an asymptomatic case [13]. Accurate diagnosis of pancreatic enlargement using various imaging modalities can avoid unnecessary surgical resection or delays in cancer treatment due to diagnostic steroid trials.
AIP had been reported in several Korean studies with retrospective report of the incidence of DM in such cases [6]. Changes of glycemic status and control methods before or after steroid treatment have not been included in Korean data. In our case, the patient was prescribed oral prednisolone 2.5 mg daily for 6 months to prevent a relapse of AIP. The duration of steroid treatment for AIP was not decided from previous reports due to relapse. An average of 47.8% Korean patients experienced relapse during a median 60 months (range, 24~197 months) of follow-up [6]. Other organ involvement or increase of IgG level was associated with relapse of AIP [6–14]. Oral corticosteroid therapy is commonly used for AIP with symptoms unless the patients have severe steroid intolerance. Several studies in the last decade have investigated the effect of corticosteroid treatment among AIP patients with DM. In a nationwide survey in Japan in 2006, 36% of patients who had DM prior to AIP onset were showed improved glycemic control after standard corticosteroid therapy [2]. These findings were supported by 3-year follow-up data revealing an improvement of glycemic control after steroid treatment in 63% of patients [5]. Hirano et al. [15] applied the glucagon tolerance test (increaments in C-peptide immunoreactivity, △CPR), insulin secretion test (HOMA-β), and insulin resistance test (HOMA-IR) in 47 patients with AIP. They assessed glucose tolerance via the △CPR test at 1 month after steroid administration, and HOMA-β and HOMA-IR were examined during the following 60 months. Glucose tolerance improved in 13% of patients and was aggravated and unchanged in 19% and 68% of patients, respectively. Insulin secretion (HOMA-β) improved significantly in 44~56% of patients, whereas insulin resistance was significantly aggravated (HOMA-IR, 1.30~1.78). Based on previous data, insulin secretion improved during steroid therapy for AIP in patients with concurrent DM. AIP patients with glucose intolerance can undergo steroid treatment at an early stage [5,15–17]. Masuda et al. [18] examined the glycemic status of 31 AIP patients who underwent steroid treatment. They concluded that new onset diabetes or worsened control of diabetes was typical in patients with an atrophic pancreas after steroid induction. However, further studies are needed to clarify the pretreatment characteristics of diabetic patients who will not benefit from steroid treatment.
In summary, AIP should be considered as a cause of significant weight loss and deterioration in glycemic control in patients with DM. Furthermore, a pancreatic imaging study should be considered in clinical practice to differentiate pancreatic cancer and AIP.
Go to : 
REFERENCES
1. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001; 344:732–8.

2. Nishimori I, Tamakoshi A, Kawa S, Tanaka S, Takeuchi K, Kamisawa T, et al. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas. 2006; 32:244–8.

3. Nishino T, Toki F, Oyama H, Shimizu K, Shiratori K. Long-term outcome of autoimmune pancreatitis after oral prednisolone therapy. Intern Med. 2006; 45:497–501.

4. Maire F, Le Baleur Y, Rebours V, Vullierme MP, Couvelard A, Voitot H, et al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol. 2011; 106:151–6.

5. Miyamoto Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, et al. Short and long-term outcomes of diabetes mellitus in patients with autoimmune pancreatitis after steroid therapy. Gut Liver. 2012; 6:501–4.

6. Lee HW, Moon SH, Kim MH, Cho DH, Jun JH, Nam K, et al. Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: long-term follow-up results after steroid therapy with short-duration maintenance treatment. J Gastroenterol. 2018; 53:967–77.

7. Yan T, Ke Y, Chen Y, Xu C, Yu C, Li Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS One. 2017; 12:e0174735.

8. Miyazawa M, Takatori H, Shimakami T, Kawaguchi K, Kitamura K, Arai K, et al. Prognosis of type 1 autoimmune pancreatitis after corticosteroid therapy-induced remission in terms of relapse and diabetes mellitus. PLoS One. 2017; 12:e0188549.

9. Cai O, Tan S. From pathogenesis, clinical manifestation, and diagnosis to treatment: an overview on autoimmune pancreatitis. Gastroenterol Res Pract. 2017; 2017:3246459.

10. The Japan Pancreas Society, the Ministry of Health and Welfare Investigation Research Team for Intractable Pancreatic Disease. Clinical diagnostic criteria for autoimmune pancreatitis 2011 (proposal). J Jpn Pancreas (Suizo). 2012. 27:17–25.
11. Motosugi U, Ichikawa T, Yamaguchi H, Nakazawa T, Katoh R, Itakura J, et al. Small invasive ductal adenocarcinoma of the pancreas associated with lymphoplasmacytic sclerosing pancreatitis. Pathol Int. 2009; 59:744–7.

12. Ranković B, Limbaeck-Stokin C, Dsokić M, Stanisavljević D, Volavšek M. Simultaneous occurrence of pancreatic mixed acinar-ductal adenocarcinoma and primary follicular lymphoma of the duodenum, accompanied by increased number of IgG4 plasma cells in tumor-free parenchyma as concomitant IgG4-related disease or reaction to tumor? A case report. Pol J Pathol. 2017; 68:86–91.

13. Okazaki K, Chari ST, Frulloni L, Lerch MM, Kamisawa T, Kawa S, et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology. 2017; 17:1–6.

14. Park SJ, Kim MH, Moon SH, Han JH, Park DH, Lee SS, et al. Clinical characteristics, recurrence features, and treatment outcomes of 55 patients with autoimmune pancreatitis. Korean J Gastroenterol. 2008; 52:230–46.
15. Hirano K, Isogawa A, Tada M, Isayama H, Takahara N, Miyabayashi K, et al. Long-term prognosis of autoimmune pancreatitis in terms of glucose tolerance. Pancreas. 2012; 41:691–5.

16. Matsubayashi H, Ishiwatari H, Imai K, Kishida Y, Ito S, Hotta K, et al. Steroid therapy and steroid response in autoimmune pancreatitis. Int J Mol Sci. 2019; 21:257.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download