RESULTS
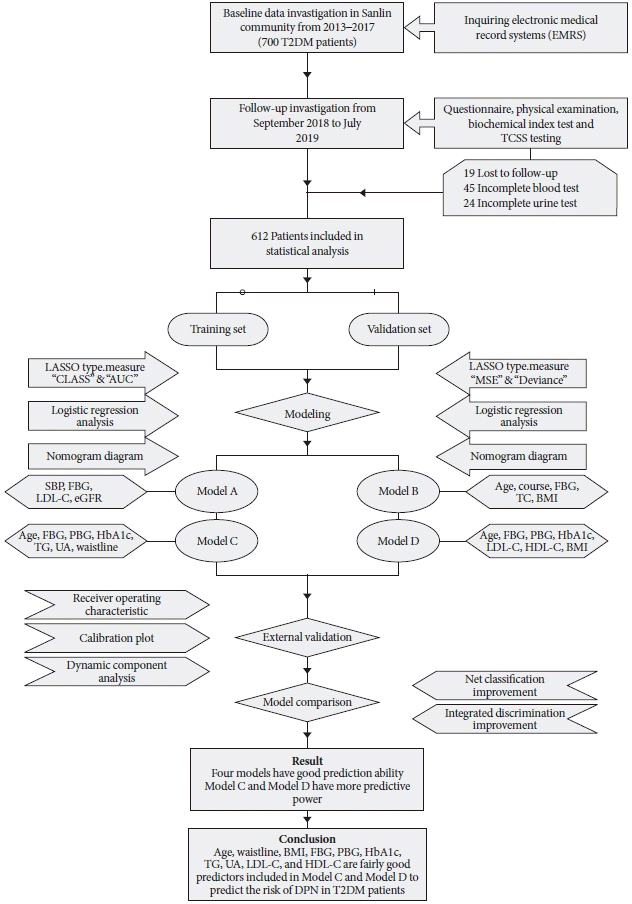
Six hundred and twelve patients with valid data were included in this study, 218 male patients and 394 female patients. The average age of the patients was 65.00 years old (range, 60.00 to 69.00). Two hundred and ninety patients (35.78%) had DPN. Patients was divided into training set (460 people) and validation set (152 people). Specific demographic and clinical characteristics are shown in
Table 1.
Table 1.
Characteristics of the participants in different groups
|
Characteristic |
Total (n=612) |
Training set (n=460) |
Validation set (n=152) |
P value |
|
Age, yr |
65.00 (60.00–69.00) |
65.00 (60.00–68.30) |
65.50 (60.00–70.00) |
0.189 |
|
Gender |
|
|
|
0.315 |
|
Male |
218 (35.62) |
169 (36.74) |
49 (32.24) |
|
|
Female |
394 (64.38) |
291 (63.26) |
103 (67.76) |
|
|
Diagnosed DPN |
219 (35.78) |
167 (36.30) |
52 (34.21) |
0.641 |
|
Course of disease, yr |
8.00 (4.00–13.00) |
8.00 (4.00–13.00) |
8.00 (4.00–13.00) |
0.921 |
|
BMI, kg/m2
|
24.80 (22.90–27.10) |
24.80 (22.70–27.10) |
24.80 (23.10–27.10) |
0.626 |
|
Waistline, cm |
86.00 (80.00–92.30) |
85.00 (79.00–93.00) |
87.00 (80.00–92.00) |
0.443 |
|
SBP, mm Hg |
137.00 (124.00–148.00) |
137.00 (124.00–148.00) |
137.00±17.10 |
0.639 |
|
DBP, mm Hg |
77.70±9.69 |
77.20±9.88 |
76.20±8.95 |
0.031 |
|
FBG, mmol/L |
6.80 (5.80–8.33) |
6.80 (5.88–8.33) |
6.90 (5.60–8.33) |
0.492 |
|
PBG, mmol/L |
11.30 (7.90–14.00) |
11.30 (7.90–13.90) |
11.80 (7.90–14.30) |
0.481 |
|
HbA1c, % |
7.10 (6.30–7.90) |
7.00 (6.30–7.80) |
7.20 (6.18–8.03) |
0.787 |
|
TC, mmol/L |
4.89±1.03 |
4.92±1.03 |
4.80±1.01 |
0.200 |
|
TG, mmol/L |
1.39 (1.02–1.95) |
1.37 (1.02–1.95) |
1.50 (0.99–1.91) |
0.567 |
|
LDL-C, mmol/L |
1.60 (1.32–1.90) |
1.62 (1.34–1.90) |
1.56 (1.29–1.88) |
0.470 |
|
HDL-C, mmol/L |
1.59 (1.38–1.89) |
1.60 (1.38–1.88) |
1.59 (1.34–1.91) |
0.731 |
|
BUN, mmol/L |
5.21 (4.39–6.16) |
5.30 (4.42–6.21) |
5.06 (4.22–6.13) |
0.971 |
|
UA, μmol/L |
294 (252–340) |
298 (252–344) |
289 (252–335) |
0.261 |
|
eGFR, mL/min |
58.00 (40.90–78.00) |
56.30 (40.50–76.10) |
60.50 (44.10–79.40) |
0.559 |

The data from the training set were analyzed using a LASSO regression analysis. AUC was used to screen four factors, including FBG, estimated glomerular filtration rate (eGFR), SBP, and LDL-C (
Fig. 2A and
E). CLASS was used to screen five factors, namely, age, body mass index (BMI), disease course, FBG, and TC (
Fig. 2B and
E). MSE was used to screed seven factors, namely, age, HbA1c, FBG, PBG, TG, waist circumference, and UA (
Fig. 2C and
E); deviance was used to screen seven factors, including age, BMI, and HbA1c, FBG, PBG, LDL-C, and HDL-C (
Fig. 2D and
E). The specific coefficients corresponding to the variables and the lambda.1se obtained for different types of measures are shown in
Supplementary Table 2. The risk factors selected were included in the logistic regression analysis. The
P values of all the included variables was less than 0.05 (
Table 2). Then, four nomogram diagrams corresponding to model A, B, C, D were established (
Fig. 3).
Fig. 2.
Demographic and clinical features selected using the Lead Absolute Shrinkage and Selection Operator (LASSO) analysis. (A) Area under curve (AUC): LASSO coefficient profiles of the four features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using fivefold cross-validation, where the optimal lambda value resulted in four features with nonzero coefficients. (B) Misclassification error (CLASS): LASSO coefficient profiles of the five features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in five features with nonzero coefficients. (C) Mean squared error (MSE): LASSO coefficient profiles of the seven features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in seven features with nonzero coefficients. (D) Deviance: LASSO coefficient profiles of the seven features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in seven features with nonzero coefficients. (E) CLASS & MSE & deviance & AUC: optimal parameters (lambda) selected in the LASSO model using five-fold cross-validation based on the minimum criteria. The partial likelihood deviance (binomial deviance) curve was plotted versus log(lambda). Dotted vertical lines were drawn at the optimal values using the minimum criteria and the 1-standard error (SE) of the minimum criteria.
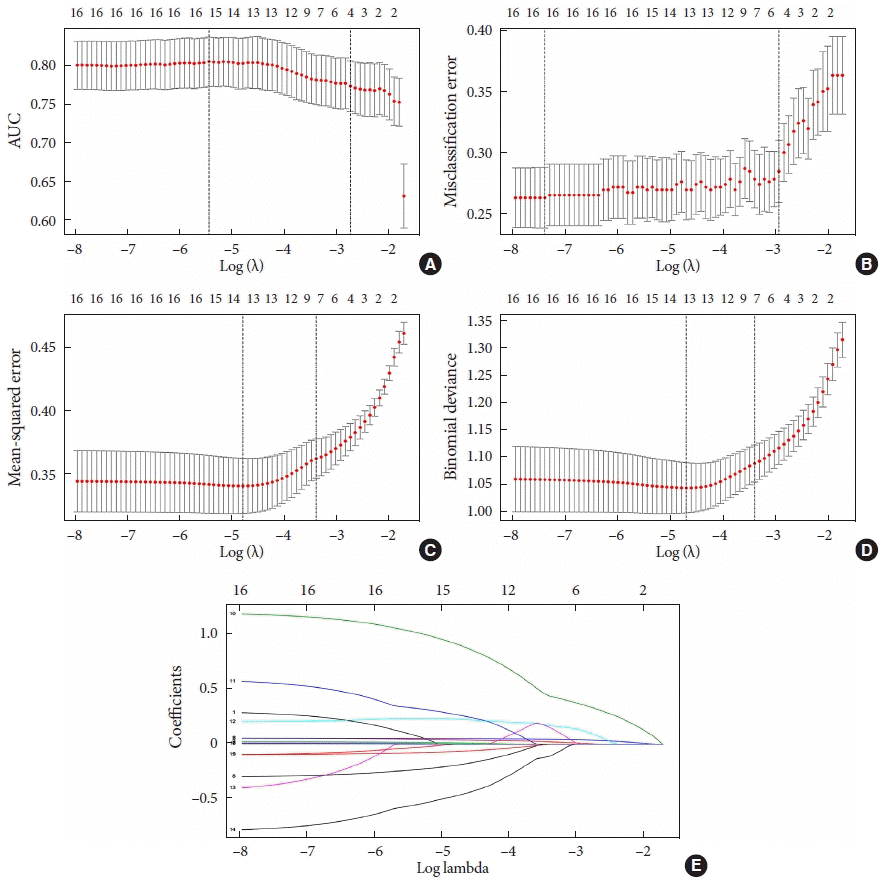

Fig. 3.
The nomograms for diabetic peripheral neuropathy (DPN) based on models A, B, C, and D. (A) Model A: The nomogram for DPN in patients with type 2 diabetes mellitus (T2DM) was developed in the cohort by integrating systolic blood pressure (SBP), fasting blood glucose (FBG) levels, low-density lipoprotein cholesterol (LDL-C) levels, and estimated glomerular filtration rate (eGFR). (B) Model B: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, disease course, FBG levels, total cholesterol (TC) levels, and body mass index (BMI). (C) Model C: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, postprandial blood glucose (PBG) levels, FBG levels, glycosylated hemoglobin (HbA1c) levels, TC levels, uric acid (UA) levels, and waist circumference. (D) Model D: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, PBG levels, FBG levels, HbA1c levels, LDL-C levels, high-density lipoprotein cholesterol (HDL-C) levels, and BMI.
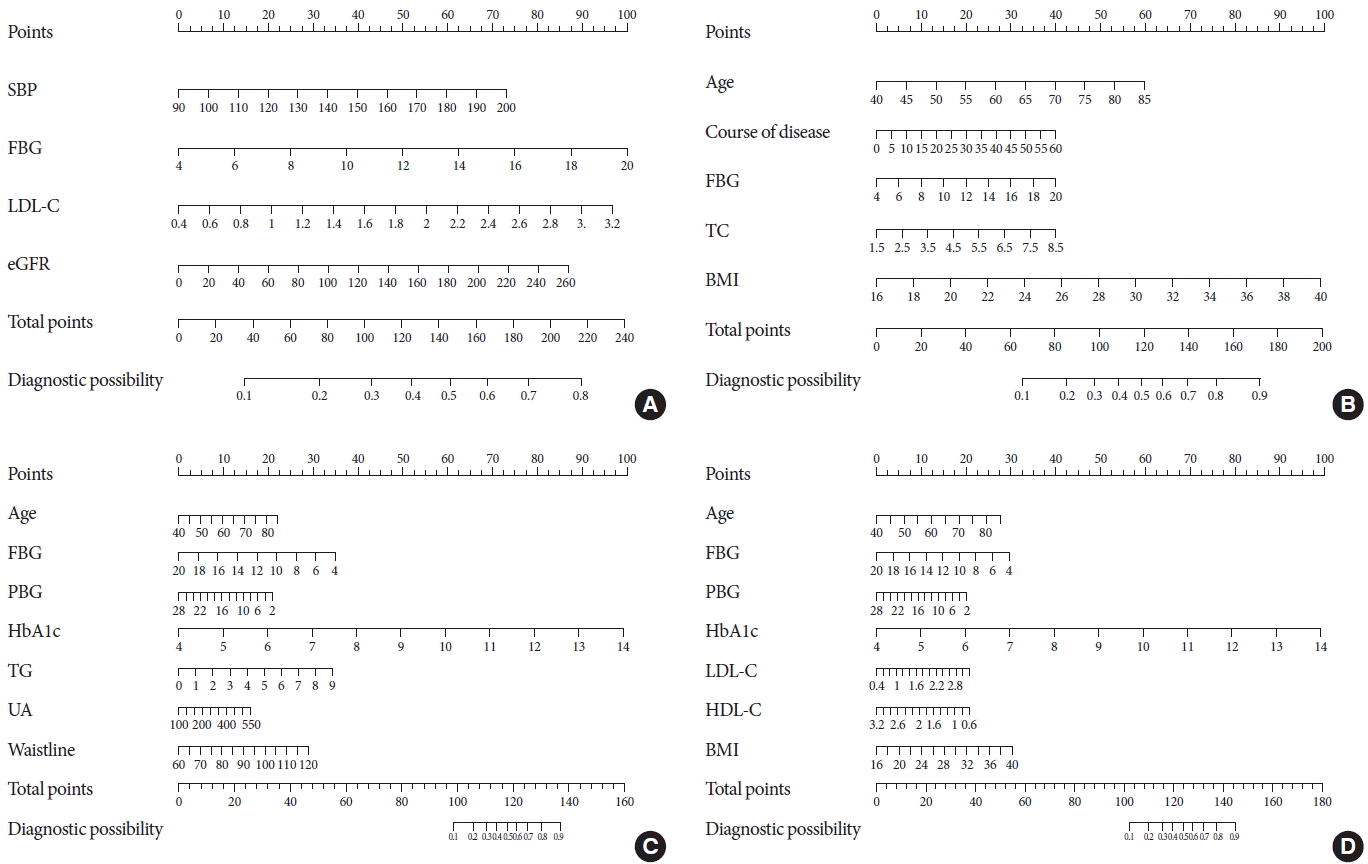

Table 2.
Models established by logistic regression analysis based on the training set
|
Factor |
β-Coefficient |
Wald-test |
P value |
OR (95% CI) |
|
Model A |
|
|
|
OR (95% CI) |
|
FBG, mmol/L |
0.123 |
2.877 |
0.004 |
1.131 (1.041–1.232) |
|
eGFR, mL/min |
0.007 |
2.076 |
0.038 |
1.007 (1.000–1.013) |
|
SBP, mm Hg |
0.013 |
2.272 |
0.023 |
1.013 (1.002–1.025) |
|
LDL-C, mmol/L |
0.687 |
3.008 |
0.003 |
1.987 (1.275–3.127) |
|
Model B |
|
|
|
|
|
Age, yr-old |
0.054 |
3.165 |
0.002 |
1.056 (1.021–1.093) |
|
BMI, kg/m2
|
0.171 |
5.088 |
<0.001 |
1.186 (1.112–1.269) |
|
Course of disease, yr |
0.040 |
2.410 |
0.016 |
1.028 (1.005–1.063) |
|
FBG, mmol/L |
0.103 |
2.217 |
0.027 |
1.108 (1.012–1.214) |
|
TC, mmol/L |
0.233 |
2.251 |
0.024 |
1.262 (1.032–1.550) |
|
Model C |
|
|
|
|
|
Age, yr-old |
0.057 |
2.874 |
0.004 |
1.058 (1.019–1.101) |
|
Waistline, cm |
0.055 |
4.049 |
<0.001 |
1.057 (1.029–1.086) |
|
HbA1c, % |
1.137 |
7.296 |
<0.001 |
3.117 (2.326–4.290) |
|
FBG, mmol/L |
–0.250 |
–3.065 |
0.002 |
7.787 (6.610–9.108) |
|
PBG, mmol/L |
–0.090 |
–2.461 |
0.014 |
0.914 (0.850–0.980) |
|
TG, mmol/L |
0.433 |
3.180 |
0.001 |
1.541 (1.193–2.037) |
|
UA, μmol/L |
0.004 |
2.223 |
0.026 |
1.004 (1.000–1.008) |
|
Model D |
|
|
|
|
|
Age, yr-old |
0.063 |
3.370 |
<0.001 |
1.065 (1.027–1.106) |
|
BMI, kg/m2
|
0.128 |
3.556 |
<0.001 |
1.137 (1.060–1.222) |
|
HbA1c, % |
1.017 |
6.856 |
<0.001 |
2.764 (2.090–3.741) |
|
FBG, mmol/L |
–0.189 |
–2.475 |
0.013 |
0.828 (0.711–0.959) |
|
PBG, mmol/L |
–0.079 |
–2.218 |
0.027 |
0.924 (0.860–0.989) |
|
LDL-C, mmol/L |
0.743 |
2.858 |
0.004 |
2.102 (1.268–3.521) |
|
HDL-C, mmol/L |
–0.797 |
–2.436 |
0.015 |
0.451 (0.235–0.848) |

The ROCs of model A, B, C, D were reported in
Supplementary Table 3 and
Supplementary Fig. 1. The ROC value of model A, B, C, D is 0.345 (0.594 to 0.683), 0.324 (0.590 to 0.760), 0.327 (0.727 to 0.796), and 0.313 (0.689 to 0.808) in training set, and the ROC value of model A, B, C, D is 0.354 (0.650 to 0.635), 0.395 (0.720 to 0.654), 0.430 (0.800 to 0.712), and 0.556 (0.910 to 0.654) in validation set. The calibration test produced S:
P values for models A, B, C, and D in the training set and validation set of 0.951, 0.983, 0.915, 0.990, and 0.987, 0.713, 0.906, 0.520, which showed in
Supplementary Fig. 2, respectively. Hosmer-Lemeshow tests were performed using the four models for the training set and validation set. In the training set, the corresponding
P values of the four models were 0.333, 0.917, 0.379, and 0.915, respectively. In the validation set, the corresponding
P values of the four models were 0.611, 0.179, 0.353, and 0.353, respectively. The
P values of the four models were greater than 0.05, indicating that these models had good fits and were valid. The DCA revealed threshold probabilities of models A, B, C, and D in the training set of 36% to 60%, 36% to 66%, 36% to 80%, and 36% to 78%, respectively (
Supplementary Fig. 2). The DCA decision curve indicated threshold probabilities of models A, B, C, and D in the validation set of 34% to 43%, 34% to 60%, 34% to 77%, and 34% to 71%, respectively (
Supplementary Fig. 2).
By calculating the NRI of the continuous variables in the training set, the cutoff was 0.327 (0.727 to 0.796), model C was better than model D, model D was better than model B, model B was better than model A (
Supplementary Fig. 3). In the validation set, the cutoff was 0.556 (0.910 to 0.654), model D was better than model C, model C was better than model B, model B was better than model A (
Supplementary Fig. 3). After calculating the IDI of the continuous variables based on the training set, model C was better than model D, model D was better than model B, model B was better than model A. Based on the validation set, model C was better than model D, model D was better than model B, model B was better than model A. Therefore, models C and D were improved compared with models A and B, indicating that the characteristic risk factors included in models C and D met the clinical prediction modeling standard (
Table 3).
Table 3.
Comparison of the prediction ability among different models through NRI and IDI
|
Variable |
Model A–B |
Model A–C |
Model A–D |
Model B–C |
Model B–D |
Model C–D |
|
NRI in training set |
|
|
|
|
|
|
|
P value |
0.004 |
0 |
0 |
<0.001 |
<0.001 |
0.681 |
|
NRI |
0.161 |
0.330 |
0.302 |
0.169 |
0.161 |
–0.013 |
|
2.5% CI |
0.051 |
0.215 |
0.191 |
0.075 |
0.071 |
–0.074 |
|
97.5% CI |
0.270 |
0.444 |
0.414 |
0.263 |
0.250 |
0.048 |
|
NRI in validation set |
|
|
|
|
|
|
|
P value |
0.017 |
<0.001 |
0 |
0.028 |
0.002 |
0.638 |
|
NRI |
0.223 |
0.348 |
0.431 |
0.193 |
0.270 |
0.039 |
|
2.5% CI |
0.040 |
0.150 |
0.271 |
0.021 |
0.103 |
–0.122 |
|
97.5% CI |
0.406 |
0.545 |
0.590 |
0.365 |
0.436 |
0.199 |
|
IDI in training set |
|
|
|
|
|
|
|
P value |
<0.001 |
0 |
0 |
0 |
0 |
0.003 |
|
IDI |
0.068 |
0.240 |
0.209 |
0.172 |
0.141 |
–0.032 |
|
2.5% CI |
0.038 |
0.196 |
0.166 |
0.133 |
0.105 |
–0.053 |
|
97.5% CI |
0.099 |
0.285 |
0.251 |
0.212 |
0.176 |
–0.011 |
|
IDI in validation set |
|
|
|
|
|
|
|
P value |
0.014 |
0 |
0 |
0 |
0 |
0.371 |
|
IDI |
0.082 |
0.272 |
0.248 |
0.190 |
0.166 |
–0.024 |
|
2.5% CI |
0.017 |
0.191 |
0.171 |
0.124 |
0.096 |
–0.077 |
|
97.5% CI |
0.146 |
0.353 |
0.325 |
0.257 |
0.237 |
0.029 |

DISCUSSION
Four DPN models were established in this study. According to the results, models C and D were more excellent models. Since the selected variables included in models A and B were all significant in the logistic regression analysis, these factors in model A and model B significantly correlated with DPN. Models C and D were validated by constructing an ROC curve and calibration curve, and were compared with NRI and IDI models, indicating that the accuracy of these two models was significantly better than model A and model B. Therefore, the influencing factors included in model C and model D are the risk factors that patients with T2DM presenting with DPN must closely monitor. According to the four models, seven factors, FBG, PBG, LDL-C, age, TC, BMI, and HbA1c, appear in two or more models and significantly modulate DPN.
Although most of the factors ultimately obtained in this study have a certain coincidence and similarity with the results of previous studies, this study differs from the perspective of statistical research methods and the previous studies using a logistic regression analysis alone. We tried to use the new statistical methods and models to study the risk factors for DPN based on previous studies and explained the problem from different perspectives. Previous researches have used a single factor analysis to validate a multivariate analysis or performed stepwise regression and recycling logistic regression analyses of the process to obtain the results. For example, Pai et al. [
6] used a multivariate logistic regression analysis to explore the risk factors for DPN in patients with T2DM by investigating the prevalence of and biochemical risk factors for DPN in patients with or without neuropathy. After adjusting for all other potential confounding factors, Khawaja et al. [
1] performed a binary logistic regression analysis to determine independent predictors of peripheral neuropathy. However, in this process, various confounding factors must be considered as variables along with the problem of multicollinearity. In the present study, the LASSO regression analysis provided a better solution to this problem with more accurate results. The greatest difference between this method and previous studies using a logistic regression analysis was that the population was randomly divided into groups at a 3:1 ratio for external verification. Variables were screened using the LASSO regression analysis, and a traditional logistic regression analysis was also performed. ROC, calibration and DCA curves were constructed for the training and validation sets to verify the accuracy and stability of the two models. NRI and IDI were introduced to compare the models and assess their stability.
In fact, the present study lacks a review of other factors contributing to DPN in patients with T2DM, including smoking, alcohol consumption, diet, other lifestyle factors, some biochemical parameters, and some pharmacological parameters. For example, a cross-sectional survey showed a strong relationship between a family history of diabetes and the development of DPN [
7]. Another survey of 2837 patients showed that insulin therapy, microalbuminuria and apparent albuminuria were independently related to DPN [
6]. The leukocyte count is also related to DPN, while oral hypoglycemia will reduce the incidence of DPN [
8]. The aforementioned factors, including smoking, alcohol consumption, and diet, were not included in the initial investigation of this study. Therefore, the team was unable to determine whether these factors would cause DPN in patients with T2DM. The research team will conduct a more detailed investigation by collecting samples and analyzing indicators in the population of patients with T2DM in the future and will further analyze the factors influencing peripheral neuropathy in Chinese patients with T2DM by including more people with T2DM and a more comprehensive list of factors.
As shown in the present study, SBP was one of the risk factors for DPN among patients withT2DM, consistent with previous studies. A systematic review showed a 2.6-fold higher SBP in T2DM patients with DPN than in T2DM patients without DPN [
9]. Regardless of whether T2DM is accompanied by hypertension, an elevated SBP always increased the risk of DPN [
10]. According to the study by Yokoyama et al. [
11], the occurrence of diabetic neuropathy is significantly correlated with SBP.
In the data analysis, FBG was one of the important factors influencing the risk of comorbid DPN in T2DM patients. A study of 110 healthy individuals, 83 T2DM patients, and 65 patients with DPN concluded that the FBG was a risk factor for DPN [
12]. Higher FBG are associated with a higher probability of developing DPN [
8].
The higher LDL-C, the greater the risk of DPN in T2DM patients [
13]. The blood viscosity of patients with diabetes increases because of the abnormal blood lipid levels, which impedes blood flow, results in the formation of a micro thrombus, and substantially affecting blood circulation [
5]. An insufficient blood supply in the nervous system leads to an energy metabolism disorder, which substantially impairs the transmission of signals in the nervous system [
14]. A study of T2DM patients in Taiwan identified elevated LDL-C as an independent risk factor for DPN [
15]. The amount of filtrate produced by both kidneys per unit time is called the eGFR, which is an indicator of renal function. This study revealed a close relationship between the eGFR and DPN. Zhang et al. [
16] analyzed 1,059 T2DM patients and observed a higher eGFR in the DPN group than in the non-DPN group. The eGFR is an important risk factor for concurrent DPN [
16]. DPN is also related to FBG, the diabetes duration and a decreased eGFR [
17].
With aging, the resistance of the human body will decrease, the level of organ function will decrease, and the incidence of many diseases will increase. A cross-sectional study identified an older age as a risk factor for DPN [
6]. A survey in Myanmar also reported an increased risk of diabetic peripheral disease with aging [
18]. Using multivariate logistic regression analysis, a survey of 248 patients with diabetes indicated that DPN was independently related to aging [
19]. Sendi et al. [
7] identified a significant correlation between DPN and increasing age. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study identified age as the most significant risk factor for DPN other than HbA1c levels [
20].
A longer disease course increased the probability of T2DM patients diagnosed with DPN. A cross-sectional study showed a positive correlation between disease course and DPN [
21]. The duration of diabetes and smoking were significant risk factors for DPN [
18]. By performing a logistic regression analysis, Khawaja et al. [
1] showed that long-standing diabetes (≥5 years of diabetes) was significantly associated with DPN.
TC is the sum of the cholesterol contained in all lipoproteins in the blood. The analysis performed in this study concluded that TC were associated with DPN. A study of 200 patients with T2DM showed a significant correlation between a TC >5.2 mmol/L and DPN [
22].
T2DM patients with DPN are at risk of developing foot ulcers, which substantially affect the quality of life of the patients. The plantar pressure is abnormal in T2DM patients presenting with DPN [
23], and the plantar pressure is related to ulcers [
24]. In other words, plantar pressure is related to the risk and severity of peripheral neuropathy. Some studies conducted in western countries have shown that obesity (BMI >30 kg/m
2) causes plantar hypertension [
25,
26]. Therefore, obese patients with T2DM are more likely to suffer from DPN. In the study by Zhen et al. [
27], the prevalence of DPN was 62.62% in obese patients with T2DM and 46.99% in patients with T2DM presenting a normal weight. The prevalence of DPN was significantly different between the two groups [
27]. The possible pathogenic mechanism is that the obese patients are too heavy, which increases the pressure on the sole of the foot. This increased pressure causes the direct mechanical destruction of the soft tissue of the foot, ischemia and necrosis due to longterm local tissue compression and repeated forces that induce inflammation [
28].
According to the results of the LASSO and logistic regression analyses, PBG are strongly correlated with the risk of DPN in T2DM patients. A related study using a multivariate logistic regression analysis of risk factors for DPN concluded that the 2-hour PBG was positively correlated with DPN [
12].
Based on the results of the present study, HbA1c were significantly related to DPN, consistent with other studies. Pai et al. [
6] noted significant differences in age, the disease course, and HbA1c between patients with and without DPN. A survey of 37,375 people concluded that T2DM patients and HbA1c greater than 7.0% had an increased risk of DPN. Both T2DM and HbA1c have a linear relationship with DPN [
21]. Another group survey also described an association of HbA1c with DPN in patients with diabetes [
26]. A study of 388 T2DM patients identified a positive correlation between HbA1c and DPN by performing a multivariate logistic regression analysis [
27]. Under normal conditions, the human body can maintain a certain blood sugar level through hormonal and nerve regulation, but under the joint actions of genetic factors and environmental factors (such as an unreasonable diet, obesity, etc.), the regulatory function will be disrupted and the blood sugar level will increase. Short-term and single hyperglycemic event do not cause serious damage to the human body. However, long-term hyperglycemia will cause pathological changes in various tissues and organs of the body, leading to acute and chronic complications, such as decreased resistance, impaired renal function, neuropathy, fundus diseases, cardiovascular and cerebrovascular diseases, and diabetic foot, among others. Therefore, effective control of HbA1c is helpful to protect against DPN in T2DM patients.
The higher the UA, the higher the risk of cooccurring DPN in T2DM patients, consistent with the results of the study by Papanas et al. [
29]. A study by Lin et al. [
22] showed a significant correlation between elevated blood UA and DPN, indicating that this parameter is a predictor of DPN. Serum UA were significantly elevated in a meta-analysis of patients with diabetes. Hyperuricemia is significantly associated with an increased risk of DPN, and hyperuricemia is associated with an increased risk of peripheral blood disorders [
30]. A positive correlation has been observed between TCSS scores and UA levels [
5]. However, further studies are needed to determine whether UA is involved in the pathogenesis of peripheral neuropathy in patients with T2DM.
This paper has suggested an association between the waist circumference and DPN. A study of diabetes in a young follow-up cohort showed that an increase in waist circumference was significantly associated with DPN [
31]. A Danish study also observed associations between a greater weight, waist circumference, and baseline BMI with DPN [
32]. An analysis of potential confounding factors for neuropathy by Aubert et al. [
33] also showed that the waist circumference was independently associated with peripheral neuropathy. Another Chinese study divided 100 middle-aged subjects into a group of healthy subjects, a group of subjects with T2DM but without DPN within the last 5 years, and a group of subjects with T2DM who were diagnosed with DPN within the last 5 years. DPN was significantly correlated with serum levels of biochemical indicators (TG and HbA1c) and anthropometric indicators (weight and waist circumference) [
34]. Oh et al. [
35] concluded that subjects with DPN had a higher BMI and greater waist circumference than subjects without DPN, suggesting an association between abdominal obesity and DPN.
Hypertriglyceridemia is a disorder in the synthesis or degradation of heterologous TG. It is an important risk factor for the occurrence of diseases related to metabolic syndrome, such as coronary heart disease, hypertension and diabetes. We concluded that the TG is one of the risk factors for DPN. A higher TG correlates with a greater risk [
15]. According to another study, hypertriglyceridemia is an independent risk factor for DPN in obese T2DM patients [
27]. Three different groups of subjects were analyzed in a study conducted in Taiwan using the percussion impact entropy index (PEIppi). A significant correlation was observed between TG and DPN [
34]. A high TG tends to cause “consistence,” namely, a change in the blood viscosity caused by a high lipid content in the blood, deposits on the blood vessel wall, and the gradual formation of small plaques known as atherosclerosis. However, the area and thickness of these massive deposits on the wall of the blood vessel will gradually increase, resulting in a decrease in the internal diameter of the blood vessel, a slower blood flow, and an acceleration of the process of blocking the blood vessel that may even interrupt the blood flow in serious cases. In addition to the interruption of blood flow, the obstruction might also cause a thrombus. If a thrombus occurs in the lower extremities, the blood flow of the extremities is not impaired, leading to necrosis. A 7-year follow-up survey of 8,379 people suggested that reducing cardiovascular risk factors may help prevent DPN. Cardiovascular risk factors, including hypertension and high TG, were positively correlated with DPN.
HDL-C correlated with DPN in the present study. Lower HDL-C correlated with a greater risk of DPN in patients with T2DM. In a comparative study by Sun et al. [
36], HDL-C in the diabetic group was lower than in the healthy group. In fact, HDL-C exerts an anti-inflammatory effect when present at normal levels. In T2DM patients, a decrease in HDL-C will reduce its anti-inflammatory effect or even promote inflammation [
37]. The specific mechanism is that the low HDL-C in T2DM patients activates monocytes and increases the secretion of tumor necrosis factor α (TNF-α). TNF-α is a key factor contributing to the development of atherosclerosis [
38]. In other words, HDL-C is transformed into atherogenic granules in patients with T2DM [
39] to modulate the cardiovascular function, subsequently causing pathological changes in the nervous system due to the lack of an energy supply.
The study still had some limitations. Regarding the samples, the T2DM patients analyzed were recruited from only one community in Shanghai, which does not represent the conditions of all T2DM patients in Shanghai. T2DM patients who were treated at the hospital or at home were unable to participate in the study. This study also lacked information on other potential risk factors for DPN, including lifestyle factors and drug indicators. The patients who were newly diagnosed with DPN based on the TCSS score may have been false positives. Because DPN is related to other complications of T2DM, the team will incorporate relevant factors associated with these diseases and strive to establish a more perfect DPN prediction model in future studies.
In the present study, models A, B, C, and D were established. Based on the NRI and IDI, model C and D are better predictive models. Thus, the influencing factors included in model C and D are more important risk factors for T2DM patients. FBG, PBG, LDL-C, age, TC, BMI, and HbA1cwere appeared in two or more models and significantly contributed to the risk of DPN.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download