1. Lee W. General principles of carotid Doppler ultrasonography. Ultrasonography. 2014; 33:11–17.

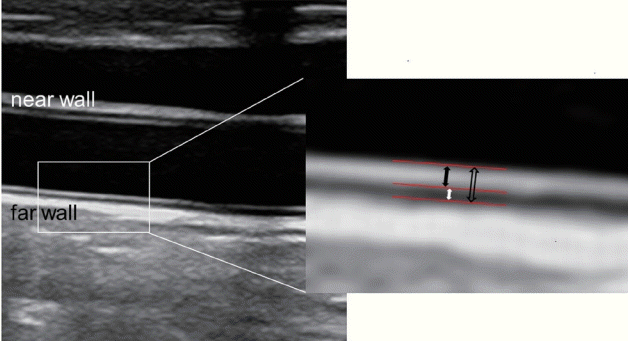
2. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34:290–296.
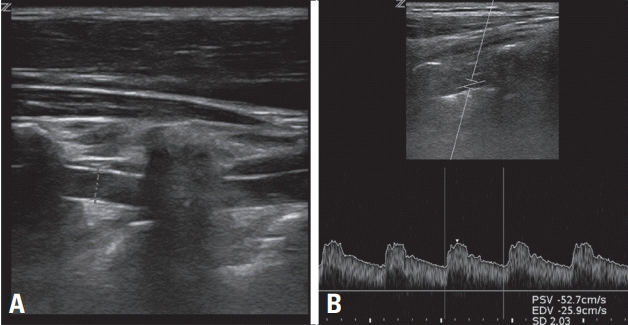
3. Lee SJ, Yu S, Hong JM, Ahn SH, Jeong SK, Lee JY, et al. Extracranial carotid duplex ultrasonography. Part I-basic principles and standard examination for carotid and vertebral arteries, and jugular veins. J Neurosonol Neuroimag. 2018; 10:47–60.
4. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008; 21:93–111. quiz 189-190.

5. Polak JF, Alessi-Chinetti JM, Kremkau FW. Doppler velocity estimates of internal carotid artery stenosis: angle correction parallel to the color Doppler Lumen versus parallel to the artery wall. J Ultrasound Med. 2019; 38:3211–3218.

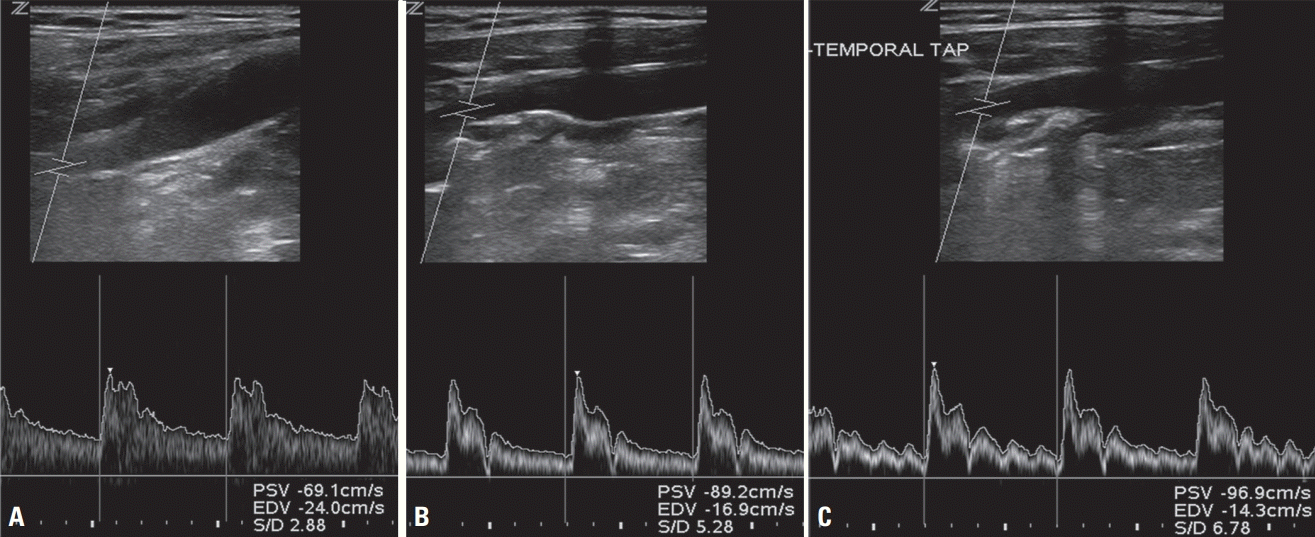
6. Kliewer MA, Freed KS, Hertzberg BS, Paulson EK, DeLong DM, Black BL, et al. Temporal artery tap: usefulness and limitations in carotid sonography. Radiology. 1996; 201:481–484.

7. Youn YJ, Lee NS, Kim JY, Lee JW, Sung JK, Ahn SG, et al. Normative values and correlates of mean common carotid intima-media thickness in the Korean rural middle-aged population: the Atherosclerosis RIsk of Rural Areas iN Korea General Population (ARIRANG) study. J Korean Med Sci. 2011; 26:365–371.

8. Lee YH, Shin MH, Kweon SS, Nam HS, Park KS, Choi JS, et al. Normative and mean carotid intima-media thickness values according to metabolic syndrome in Koreans: the Namwon study. Atherosclerosis. 2014; 234:230–236.

9. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--society of radiologists in ultrasound consensus conference. Radiology. 2003; 229:340–346.

10. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, et al. Editor’s choice - management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018; 55:3–81.

11. Jahromi AS, Cinà CS, Liu Y, Clase CM. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg. 2005; 41:962–972.

12. Stojanov D, Ilic M, Bosnjakovic P, Zivkovic M, Jolic S, Vukasinovic N, et al. New ischemic brain lesions on diffusion-weighted MRI after carotid artery stenting with filter protection: frequency and relationship with plaque morphology. AJNR Am J Neuroradiol. 2012; 33:708–714.

13. Madycki G, Staszkiewicz W, Gabrusiewicz A. Carotid plaque texture analysis can predict the incidence of silent brain infarcts among patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg. 2006; 31:373–380.

14. Lee JY, Choi HY, Lee SI, Hwang YH, Cho AH, Seo WK, et al. Extracranial carotid duplex ultrasonography. Part II-clinical utility of carotid duplex ultrasound. J Neurosonol Neuroimag. 2018; 10:61–79.
15. Stilo F, Montelione N, Calandrelli R, Distefano M, Spinelli F, Di Lazzaro V, et al. The management of carotid restenosis: a comprehensive review. Ann Transl Med. 2020; 8:1272.

16. Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004; 40:1126–1135.

17. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011; 124:489–532.
18. Aburahma AF. Duplex criteria for determining ≥50% and ≥80% internal carotid artery stenosis following carotid endarterectomy with patch angioplasty. Vascular. 2011; 19:15–20.

19. Zierler RE, Jordan WD, Lal BK, Mussa F, Leers S, Fulton J, et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg. 2018; 68:256–284.

20. Zhou W, Felkai DD, Evans M, McCoy SA, Lin PH, Kougias P, et al. Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg. 2008; 47:74–80.

21. AbuRahma AF, Abu-Halimah S, Bensenhaver J, Dean LS, Keiffer T, Emmett M, et al. Optimal carotid duplex velocity criteria for defining the severity of carotid in-stent restenosis. J Vasc Surg. 2008; 48:589–594.

22. Lal BK, Hobson RW 2nd, Tofighi B, Kapadia I, Cuadra S, Jamil Z. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008; 47:63–73.

23. Bandyk DF. Follow-up after carotid endarterectomy and stenting: What to look for and why. Semin Vasc Surg. 2020; 33:47–53.

24. den Hartog AG, Achterberg S, Moll FL, Kappelle LJ, Visseren FL, van der Graaf Y, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke. 2013; 44:1002–1007.

25. icolaides AN, Kakkos SK, Griffin M, Sabetai M, Dhanjil S, Tegos T, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg. 2005; 30:275–284.
26. Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med. 1997; 127:13–20.

27. Cheng TW, Pointer KE, Gopal M, Farber A, Jones DW, Eberhardt RT, et al. Natural history of non-operative management in asymptomatic patients with 70%-80% internal carotid artery stenosis by duplex criteria. Eur J Vasc Endovasc Surg. 2020; 60:339–346.

28. Brinjikji W, Rabinstein AA, Lanzino G, Murad MH, Williamson EE, DeMarco JK, et al. Ultrasound characteristics of symptomatic carotid plaques: a systematic review and meta-analysis. Cerebrovasc Dis. 2015; 40:165–174.

29. Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg. 2013; 57:609–618.e1. discussion 617-8.

30. US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Screening for asymptomatic carotid artery stenosis: US preventive services task force recommendation statement. JAMA. 2021; 325:476–481.
31. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012; 379:2053–2062.

32. Sturzenegger M, Mattle HP, Rivoir A, Baumgartner RW. Ultrasound findings in carotid artery dissection: analysis of 43 patients. Neurology. 1995; 45:691–698.

33. Yang L, Ran H. Extracranial vertebral artery dissection: findings and advantages of ultrasonography. Medicine (Baltimore). 2018; 97:e0067.
34. Benninger DH, Baumgartner RW. Ultrasound diagnosis of cervical artery dissection. Front Neurol Neurosci. 2006; 21:70–84.

35. Bennani H, Alami B, Hajjar C, Quenum L, Haloua M, Boubbou M, et al. Symptomatic carotid web: about a rare ultrasound finding. J Med Vasc. 2020; 45:284–287.

36. Nezu T, Hosomi N. Usefulness of carotid ultrasonography for risk stratification of cerebral and cardiovascular disease. J Atheroscler Thromb. 2020; 27:1023–1035.

37. Park HK, Hong KS. Carotid web: under-recognized etiology for ischemic stroke. J Neurosonol Neuroimag. 2018; 10:100–105.

38. Kwon JA, Gwak DS, Shim DH, Kim YW, Hwang YH. Cryptogenic stroke caused by a carotid web with a superimposed thrombosis: serial neurosonologic findings. J Neurosonol Neuroimag. 2019; 11:158–161.

39. Kliewer MA, Hertzberg BS, Kim DH, Bowie JD, Courneya DL, Carroll BA. Vertebral artery Doppler waveform changes indicating subclavian steal physiology. AJR Am J Roentgenol. 2000; 174:815–819.

40. Lee JY. Duplex ultrasonography in vertebrobasilar system. Journal of Neurosonology. 2009; 1:14–18.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download