This article has been
cited by other articles in ScienceCentral.
Abstract
We present a patient with a primitive neuroectodermal tumor arising from the right atrium who experienced multiple syncope episodes daily, which had first appeared 1 month after surgery. The symptoms continued to worsen over the course of chemotherapy, and the autonomic function test (AFT) was performed after the 14th chemotherapy cycle. The AFT revealed orthostatic hypotension and reduced baroreflex-dependent sympathetic reactivity. Physical counterpressure techniques were applied with a visual biofeedback intervention, and were found to be effective in reducing the syncope episodes.
Go to :

Keywords: Autonomic nervous system disease, Neuroectodermal tumors, Primitive, Peripheral, Orthostatic hypotension
Primitive neuroectodermal tumors (PNETs) are aggressive malignant tumors caused by a balanced reciprocal translocation t(11; 22) involving the EWSR1 and FL1-1 genes in pluripotent neural crest cells.
1 Primary tumors arising from the right atrium are extremely rare.
2 Surgical resection of a PNET with adjuvant chemotherapy generally produces a good prognosis.
3 We present a patient who was referred for evaluation of syncope due to experiencing frequent episodes of loss of consciousness. The autonomic function test (AFT) was performed to assess the parasympathetic and sympathetic reactivity. Physical counterpressure techniques were applied in the form of biofeedback for the management of syncope.
4
CASE
A 23-year-old female presented with a 6-week history of palpitation and breathlessness. Contrast computed tomography angiography (
Fig. 1) revealed an anterior mediastinal large soft-tissue mass (10.2 × 8.2 × 8.2 cm) arising from the junction of the right atrium and superior vena cava (SVC). The right atrium and ventricle were small, and the inferior vena cava appeared dilated due to compression by the mass. Functionally the heart appeared normal on echocardiography, and the metastatic workup was negative. The histopathological evaluation was suggestive of PNET expressing CD99 and FL1-1. The tumor was positive for EWSR1 translocation, confirming a diagnosis of PNET. Median sternotomy, anterior pericardiectomy, and dissection of the aorta and pulmonary artery were performed, and an intrapericardial mass was dissected from the right atrium-SVC junction. A bovine pericardial patch was placed to close the defect. Episodes of syncope appeared at 1 month after surgery, before chemotherapy was started. Fourteen cycles of the standard regimen of doxorubicin, vincristine, cyclophosphamide, and dactinomycin alternating with courses of ifosfamide and etoposide were given over a period of 1 year.
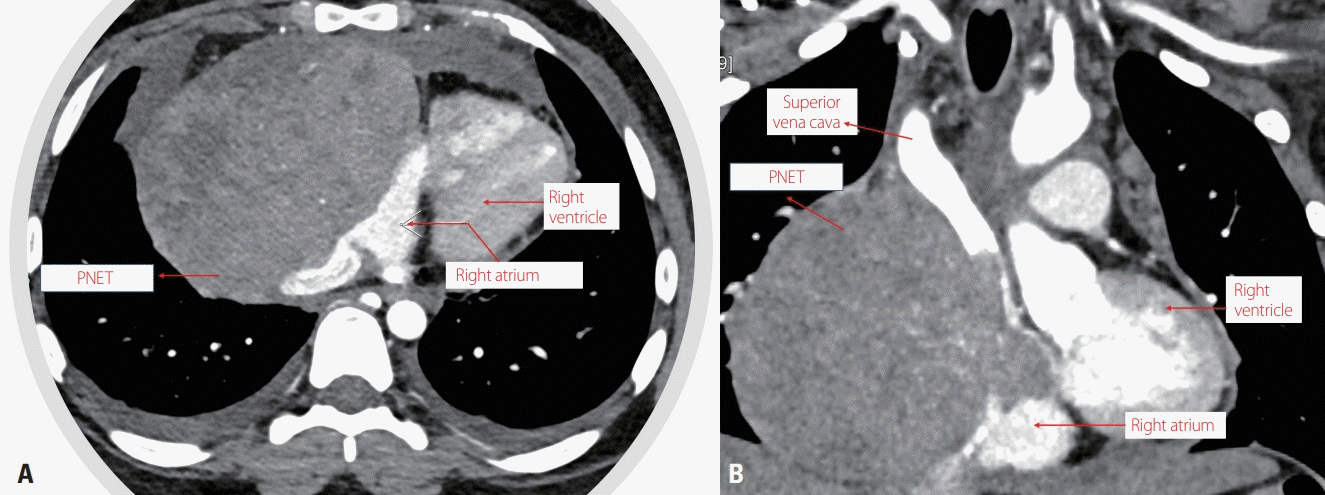 | Fig. 1.Computed tomography coronary angiography: (A) axial view and (B) coronal view. A large well-defined heterogeneously enhanced soft-tissue mass is seen on the right side of the anterior mediastinum with loss of fat planes and compression of the right atrium and ventricle. The lesion is compressing the inferior vena cava and the lower part of the superior vena cava, with invasion into the right atrium. PNET, primitive neuroectodermal tumor. 
|
At the end of the 14th cycle of chemotherapy, the syncope episodes gradually increased to 15-20 per day. Usually one episode of syncope lasted 1-2 minutes and was associated with prodromal symptoms of lightheadedness and headache. The syncope was triggered by a change in posture from supine to sitting or standing. It was not associated with emotional states, urination, defecation, or abrupt neck movement. There was no evidence of metastasis on positron-emission tomography. A postsurgery cardiac evaluation by electrocardiography (ECG) produced no abnormal findings. Normal findings for the nerve conduction velocity ruled out the possibility of vincristine-induced neuropathy. The possibility of functional disorder was ruled out by a psychiatric evaluation.
Since the symptoms of the patient continued to aggravate, she was referred to the AFT laboratory for autonomic evaluation and the first recordings were made on the same day. ECG, stethography, grip force, and beatto-beat blood pressure were recorded digitally using Powerlab and Finapress devices (ADInstruments), respectively. Follow-up recordings were made after 2 months and 4 months. In all three recordings, p-waves were predominantly inverted with different PR intervals on lead II. In the head-up tilt (HUT) test, a decrease in systolic blood pressure (SBP) of > 20 mmHg was recorded within 3 minutes after reaching 70°, which was suggestive of orthostatic hypotension and loss of baroreflex-dependent sympathetic reactivity. However, in a cold pressor test (CPT), when the patient’s hand was dipped in 10°C water, the diastolic blood pressure increased by > 15 mmHg in all recordings, suggesting normal baroreflex-independent sympathetic reactivity. Parasympathetic reactivity could not be evaluated due to the presence of ectopic beats. These findings were consistent with the presence of neurogenic orthostatic hypotension. In view of symptomatic autonomic neuropathy, the subsequent chemotherapy was completed without vincristine. Due to the worsening renal function and frequent urinary tract infection, we were advised to manage syncope using nonpharmacological methods, such as increasing salt and water intakes, physical counterpressure techniques, compression stockings, and abdominal binders.
To demonstrate the effect of applying counterpressure techniques to the patient, the HUT test was performed after instructing the patient to press against the footrest at the time of symptom onset and sustain this pressure when the symptoms aggravated (
Fig. 2). She was instructed to perform physical counterpressure techniques such muscle tensing, ball compression, and leg crossing,
4 and the HUT test was repeated. Changes in SBP were displayed on a computer monitor to provide visual biofeedback to the patient, and she was instructed to practice the techniques at home whenever she experienced presyncopal symptoms. These syncope simulation sessions were repeated multiple times. After 2 months, the frequency of syncope episodes reduced to 5-10 per day.
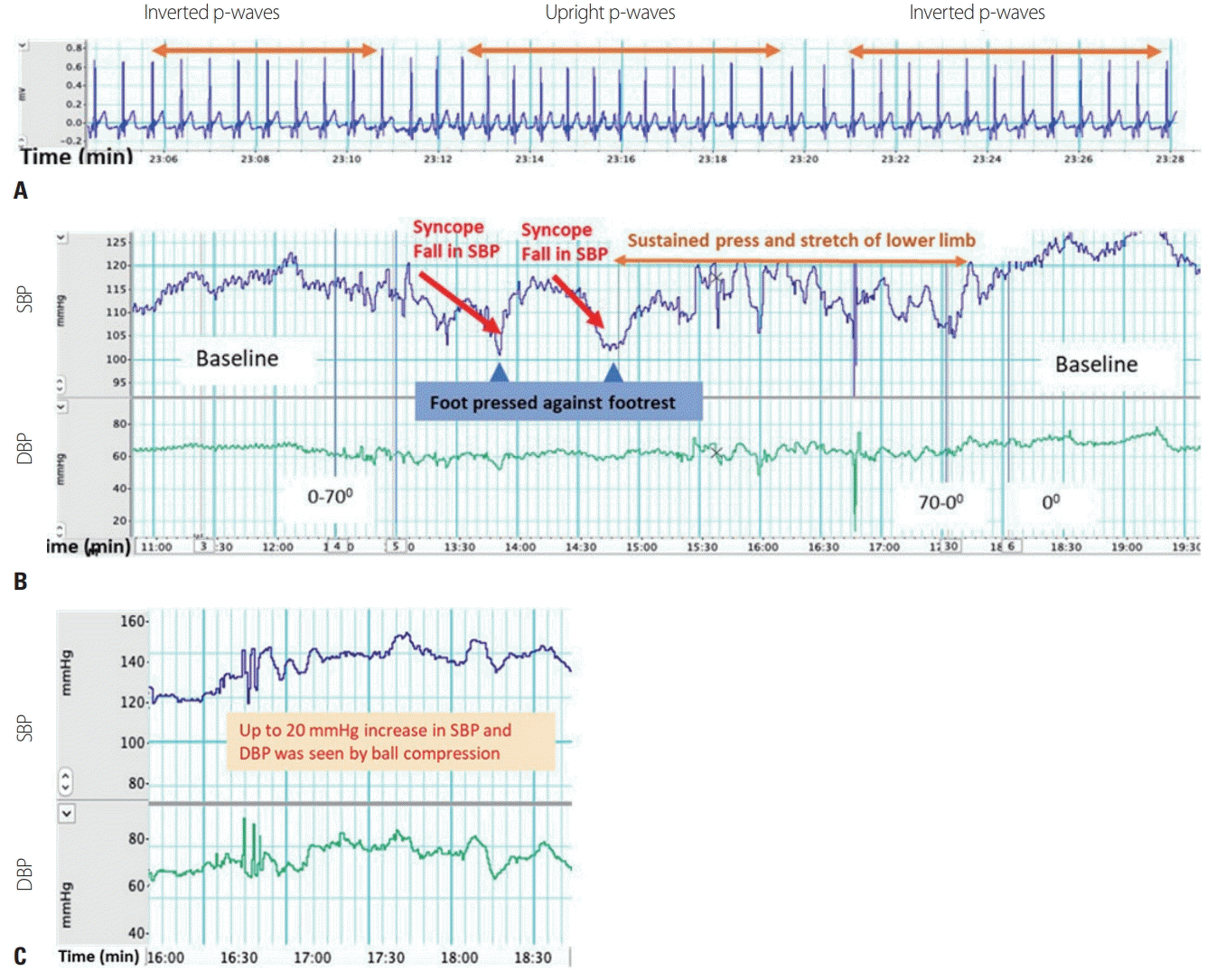 | Fig. 2.(A) Echocardiography recording showing the presence of multiple rhythm-generating foci in the right atrium. (B) Biofeedback therapy during the head-up tilt (HUT) test by sustained pressing and stretching of the lower limb. (C) Biofeedback therapy during the HUT test using a ball compression technique. DBP, diastolic blood pressure; SBP, systolic blood pressure. 
|
Go to :

DISCUSSION
This was a rare case of PNET arising from the right atrium, for which there are few published reports.
2,
5,
6 This is also the only report mentioning syncope after surgery and its management by visual biofeedback. ECG showed normal rate and rhythm with normal morphology before surgery. After surgical resection of the tumor and chemotherapy, arrhythmia and abnormal p-wave morphology were noted. This could be attributed to damage to the pacemaker cells in the right atrium during surgical resection of the tumor, but ECG showed normal cardiac hemodynamic functionality. The standard regimen of chemotherapy was used, for which there are no reports on the occurrence of arrhythmias or autonomic dysfunction in the literature.
2,
7,
8 Among the sympathetic reactivity tests, dysfunction was noted in the HUT test whereas the CPT findings were normal. Orthostatic hypotension and loss of baroreflex-dependent sympathetic reactivity were noted on all three recordings. This indicates that the baroreflex-dependent sympathetic reactivity was affected while the baroreflex-independent pathways were unaffected.
9 Damage to baroreceptors located on the aorta, right atrium, and other vessels during the surgery and several months of bed rest during the course of treatment might have reduced the baroreflex sensitivity. During increased demand or stress, a ventilation perfusion mismatch could lead to reduced oxygenation of the brain, possibly leading to syncope. The patient was instructed to perform physical counterpressure techniques such as leg crossing, isometric handgrip, and arm tensing recommended by American Heart Association and American Red Cross,
10 with the aid of a visual biofeedback intervention. These techniques rely on baroreflex-independent pathways to increase the blood pressure. We found that an increase of up to 20 mmHg could be expected with this technique. The biofeedback therapy helped our patient to understand the techniques, improved her compliance, and reduced the frequency of syncope episodes.
Go to :

Notes
Go to :

ACKNOWLEDGMENTS
The study was done in Autonomic Function Testing Lab, Department of Physiology, AIIMS.
Go to :

REFERENCES
1. Beşirli K, Arslan C, Tüzün H, Oz B. The primitive neuroectodermal tumor of the heart. Eur J Cardiothorac Surg. 2000; 18:619–621.

2. Ushigusa J, Mukae Y, Takamatsu M, Nogami E, Furutachi A, Itoh M, et al. Adult-onset primary Ewing’s sarcoma of the right atrium: a case report. Surg Case Rep. 2019; 5:171.

3. Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003; 348:694–701.

4. Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR. Physical countermeasures to increase orthostatic tolerance. J Intern Med. 2015; 277:69–82.

5. Higgins JC, Katzman PJ, Yeager SB, Dickerman JD, Leavitt BJ, Tischler MD, et al. Extraskeletal Ewing’s sarcoma of primary cardiac origin. Pediatr Cardiol. 1994; 15:207–208.

6. Cai CQ, Zhang QJ, Shen CH, Hu XL. Primary intraspinal primitive neuroectodermal tumor: a case report and review of literature. J Pediatr Neurosci. 2008; 3:154–156.

7. Nwaejike N, Rassl D, Ford H, Large SR. Primitive neuroectodermal tumor of the heart. Ann Thorac Surg. 2012; 93:e27–e29.

8. Demir A, Gunluoglu MZ, Dagoglu N, Turna A, Dizdar Y, Kaynak K, et al. Surgical treatment and prognosis of primitive neuroectodermal tumors of the thorax. J Thorac Oncol. 2009; 4:185–192.

9. Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol (Oxf). 2011; 203:37–45.

10. Charlton NP, Pellegrino JL, Kule A, Slater TM, Epstein JL, Flores GE, et al. 2019 American Heart Association and American Red Cross Focused Update for First Aid: presyncope: an update to the American Heart Association and American Red Cross Guidelines for First Aid. Circulation. 2019; 140:e931–e938.

Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download