Introduction
Materials and methods
1. Patients and study design
2. Statistical methods
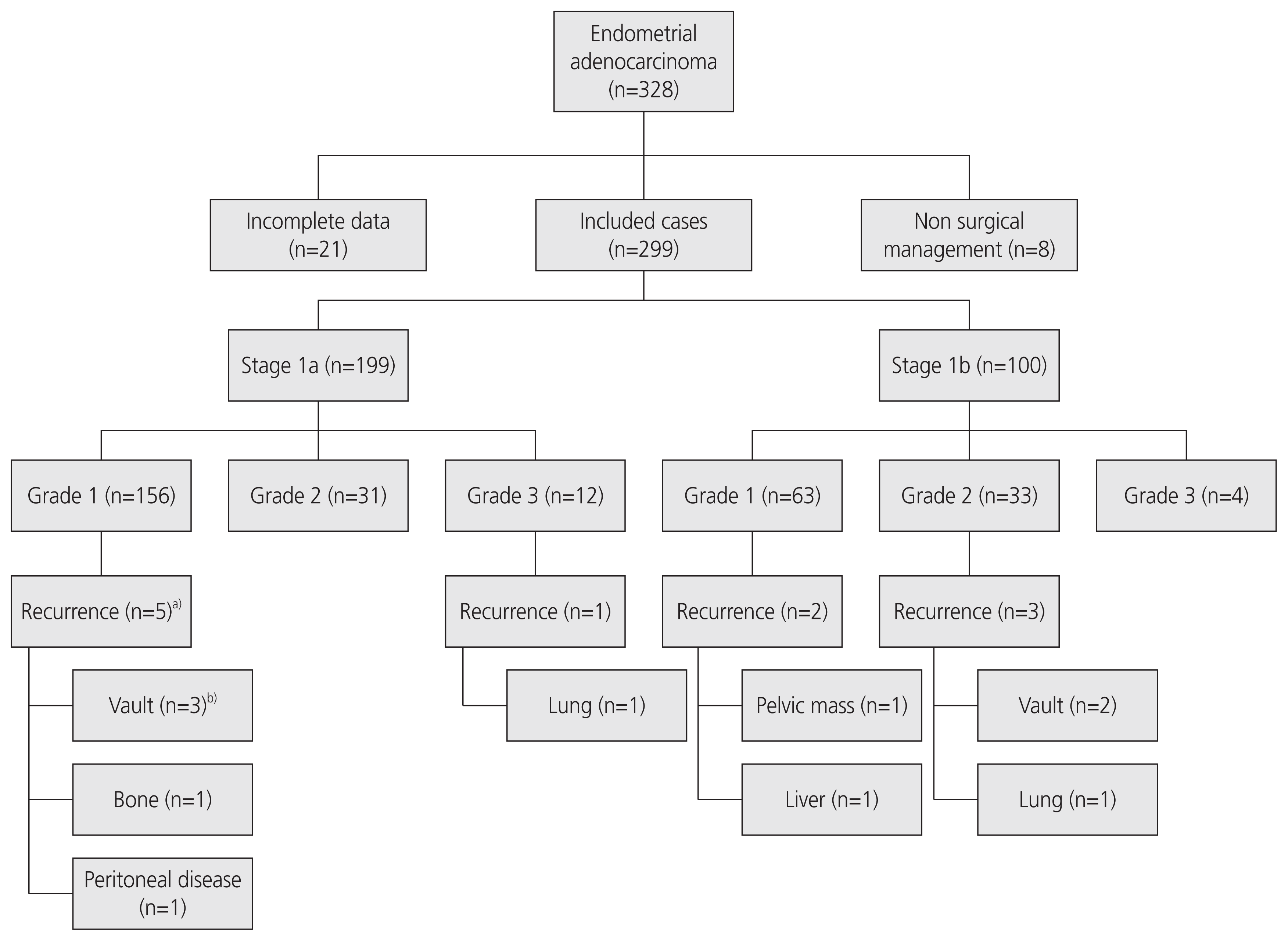
Results
Table 1
| Characteristic | Value |
|---|---|
| Parity | |
| Nulliparous | 54 (18.1) |
| Parous | 236 (78.9) |
| Unknown | 9 (3) |
| Menopausal status | |
| Pre-menopausal | 36 (12.1) |
| Post-menopausal | 259 (86.6) |
| Unknown | 4 (1.3) |
| BMI>30 | |
| Yes | 164 (54.8) |
| No | 71 (23.7) |
| Unknown | 55 (21.5) |
| Diabetic | |
| Yes | 57 (19.1) |
| No | 197 (65.9) |
| Unknown | 40 (15) |
| Hypertensive | |
| Yes | 161 (53.8) |
| No | 111 (37.1) |
| Unknown | 28 (9.1) |
| History of breast cancer | 15 (5.0) |
| Use of tamoxifen | 11 (3.7) |
| HRT | |
| Current use of HRT | 12 (4.0) |
| Past use of HRT | 25 (8.4) |
| Never used | 164 (44.8) |
| Non-applicable | 36 (12.0) |
| Unknown | 92 (30.8) |
| Presentation of original disease | |
| Post-menopausal bleeding | 231 (77.3) |
| Irregular bleeding/menorrhagia | 34 (11.4) |
| Vaginal discharge | 7 (2.3) |
| Incidental finding | 7 (2.3) |
| Weight loss | 2 (0.6) |
| Abdominal pain | 3 (1.0) |
| Unknown | 15 (5.1) |
| Stage | |
| 1a | 199 (66.5) |
| 1b | 100 (33.5) |
| Grade | |
| 1 | 219 (73.2) |
| 2 | 64 (21.4) |
| 3 | 16 (5.4) |
| Primary surgery | |
| Laparoscopic hysterectomy & bilateral salpingo-oophorectomy | 147 (49.2) |
| Abdominal hysterectomy & bilateral salpingo-oophorectomy | 150 (50.1) |
| Vaginal hysterectomy +second procedure bilateral salpingo-oophorectomy | 2 (0.7) |
| Adjuvant treatment | |
| Intravaginal radiotherapy | 64 (21.4) |
| External beam radiotherapy | 6 (2.0) |
| Intravaginal and external radiotherapy | 8 (2.7) |
| Intravaginal radiotherapy and chemotherapya) | 1 (0.3) |
| Chemotherapyb) | 1 (0.3) |
| None | 217 (72.6) |
| Radiotherapy declined by patient | 1 (0.3) |
| Patient unfit for radiotherapy | 1 (0.3) |
Table 2
| Patient (age at diagnosis) | Primary stage | Primary grade | Primary treatment | Adjuvant treatment | Recurrence presentation | Time from surgery to recurrence (mo) | Treatment for recurrence | Status at last follow up |
|---|---|---|---|---|---|---|---|---|
| 1 (81 years) | 1a | 1a) |
TAH/BSO No LVSI Tumor size: 2.3 cm Serosal clearance: 6 mm |
None | 1st recurrence: vaginal bleeding due to vault recurrence | 7 | Colpectomy, external beam radiation and brachytherapy | Alive and disease free |
| 2nd recurrence: right groin mass | 35 | Radical excision of groin mass, Provera | ||||||
| 2 (88 years) | 1a | 3 |
TLH/BSO No LVSI Tumor size: 1.4 cm Serosal clearance: 7 mm |
Noneb) | Shortness of breath, pleural effusion and lung metastases | 5 | Palliative Died of recurrence | |
| 3 (69 years) | 1a | 1 |
TAH/BSO No LVSI Tumor size: 2.9 cm Serosal clearance: 4.5 mm |
None | 1st recurrence: vaginal bleeding due to vault recurrence | 10 | External beam radiation and brachytherapy | Died of recurrence |
| 2nd recurrence: vaginal and rectal bleeding | 28 | Pelvic exenteration and chemotherapy | ||||||
| 4 (63 years) | 1a | 1 |
TLH/BSO No LVSI Tumor size: 1.8 cm Serosal clearance: 10 mm |
None | Severe hip pain due to bone metastases with enlarged lymph nodes on ultrasound | 44 | Palliative, biopsy not possible | Died of recurrence |
| 5 (74 years) | 1b | 1 |
TAH/BSO No LVSI Tumor size: 3.3 cm Serosal clearance: 1.2 mm |
Intravaginal radiotherapy | Abdominal pain, bloating and fatigue, CT showed enlarged pelvic lymph nodes, pelvic peritoneal mass and hydroureter | 14 | Chemotherapy | Died of recurrence |
| 6 (77 years) | 1b | 1 |
TLH/BSO No LVSI Tumor size: 3 cm Serosal clearance: 3.5 mm |
Intravaginal radiotherapy | Jaundice and liver metastases | 14 | Palliative | Died of recurrence |
| 7 (58 years) | 1b | 2 |
TAH/BSO No LVSI Tumor size: 4.6 cm Serosal clearance: 4 mm |
Nonec) | 1st recurrence: vaginal bleeding due to vault recurrence | 22 | Colpectomy and intravaginal radiotherapy | Died of recurrence |
| 2nd recurrence: rectal bleeding, weight loss, recurrence with rectal mass and liver metastases | 54 | Palliative | ||||||
| 8 (72 years) | 1b | 2 |
TLH/BSO LVSI positive Tumor size: 3.9 cm Serosal clearance: 4 mm |
Intravaginal radiotherapy | Shortness of breath on exertion, imaging showed solitary lung metastasis | 50 | Left upper lobectomy and Letrozole | Alive and disease free |
| 9 (68 years) | 1b | 2 |
TAH/BSO No LVSI Tumor size: 4 cm Serosal clearance: 3.6 mm |
Intravaginal radiotherapy | Vaginal bleeding | 60 | Unknown | Died-unknown cause |
| 10 (68 years) | 1a | 1 |
TAH/BSO No LVSI Tumor size: 3.2 cm Serosal clearance not measured (no myometrial involvement) |
None | Admitted with stroke Recurrence identified on CT in the form of pelvic peritoneal disease | 57 | Palliative care Megestrol and Zoladex PS: 3 | Alive |
| 11 (60 years) | 1a | 1 |
TAH/BSO No LVSI Tumor size: 2.9 cm Serosal clearance: 5.2 mm |
Incidental, MRI for hip pain showed vault recurrence, CT showed lung metastasis | 31 | Palliative chemotherapy | Alive |
Tumor size is the largest of the three dimensions documented in histopathological examination reports. Cytological examination of peritoneal washings was negative for malignant cells in all cases.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download