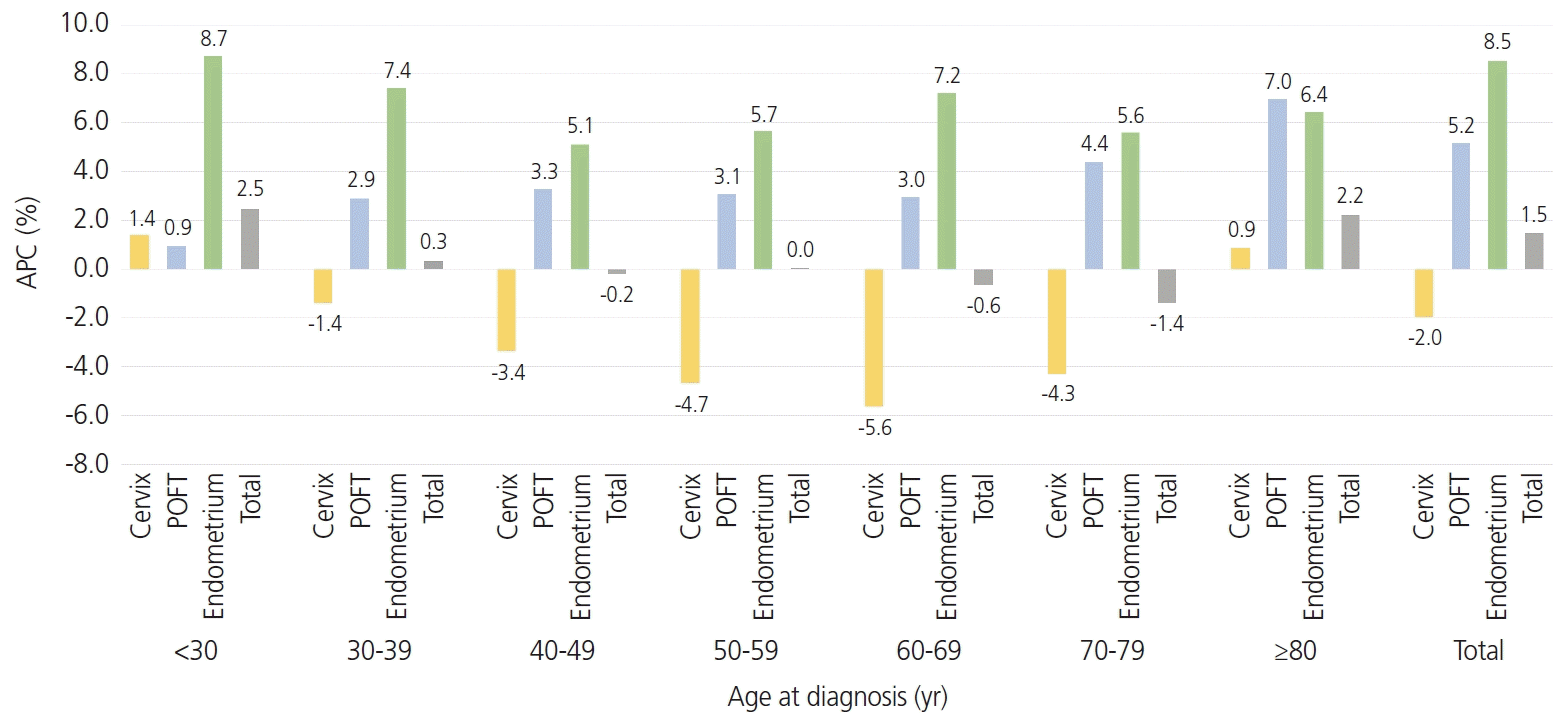
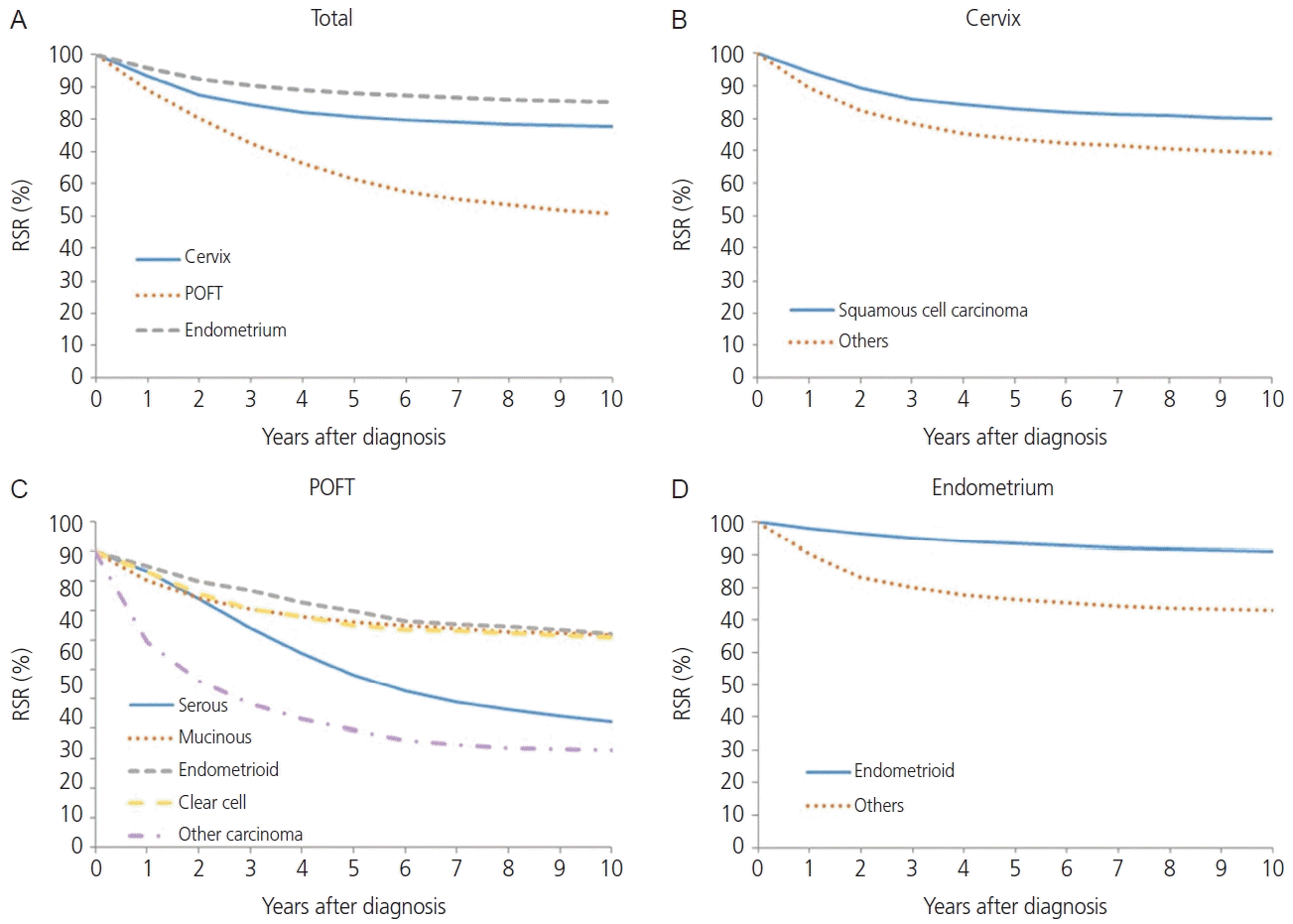
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–50.

2. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31.

3. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, et al. Cervical cancer incidence and survival in Korea: 1993-2002. Int J Gynecol Cancer. 2006; 16:1833–8.

4. Chung HH, Hwang SY, Jung KW, Won YJ, Shin HR, Kim JW, et al. Ovarian cancer incidence and survival in Korea: 1993-2002. Int J Gynecol Cancer. 2007; 17:595–600.

5. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.

6. World Health Organization. International statistical classification of diseases and related health problems. 10th ed. Geneva: World Health Organization Press;1994.
7. Segi M, Fujisaku S. Cancer mortality for selected sites in 24 countries (1950-1957). Sendai: Tohoku University School of Medicine;1960.
8. National Cancer Institute. SEER Summary staging manual 2000: codes and coding instructions. Bethesda: National Cancer Institute;2000.
9. Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Bethesda: National Cancer Institute;1959.
10. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Jun JK, et al. Trends in the incidence of in situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. PLoS One. 2013; 8:e72012.

11. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013; 24:298–302.

12. Patel A, Galaal K, Burnley C, Faulkner K, Martin-Hirsch P, Bland MJ, et al. Cervical cancer incidence in young women: a historical and geographic controlled UK regional population study. Br J Cancer. 2012; 106:1753–9.

13. Kim CJ, Kim BG, Kim SC, Kim YT, Kim YM, Park SY, et al. Sexual behavior of Korean young women: preliminary study for the introducing of HPV prophylactic vaccine. Korean J Gynecol Oncol. 2007; 18:209–18.

14. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009; 20:1–7.

15. Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012; 55:3–23.
16. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003; 348:1625–38.

17. Lee JY, Kim S, Kim YT, Lim MC, Lee B, Jung KW, et al. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer. 2018; 18:601.

18. Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016; 27:e5.

19. Baik I. Forecasting obesity prevalence in Korean adults for the years 2020 and 2030 by the analysis of contributing factors. Nutr Res Pract. 2018; 12:251–7.

20. Wise MR, Jordan V, Lagas A, Showell M, Wong N, Lensen S, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol. 2016; 214:689.e1-17.
21. Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019; 4:e137–47.

22. Lee DY, Lee TS. Associations between metabolic syndrome and gynecologic cancer. Obstet Gynecol Sci. 2020; 63:215–24.

23. Song JE, Ahn JA, Lee SK, Roh EH. Factors related to low birth rate among married women in Korea. PLoS One. 2018; 13:e0194597.

24. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019; 145:1719–30.

25. Park HJ, Hong YH, Cho YJ, Lee JE, Yun JM, Kwon H, et al. Trends and cut-point changes in obesity parameters by age groups considering metabolic syndrome. J Korean Med Sci. 2018; 33:e47.

26. Bjørge T, Engeland A, Tretli S, Weiderpass E. Body size in relation to cancer of the uterine corpus in 1 million Norwegian women. Int J Cancer. 2007; 120:378–83.

27. Parsons LHP, Pedersen R, Richardson DL, Kho KA. The prevalence of occult endometrial cancer in women undergoing hysterectomy for benign indications. Eur J Obstet Gynecol Reprod Biol. 2018; 223:108–12.

28. Desai VB, Wright JD, Gross CP, Lin H, Boscoe FP, Hutchison LM, et al. Prevalence, characteristics, and risk factors of occult uterine cancer in presumed benign hysterectomy. Am J Obstet Gynecol. 2019; 221:39.e1-14.

29. Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017; 10:CD007585.