1. Arnautović KI, Al-Mefty O, Angtuaco E, Phares LJ. Dural arteriovenous malformations of the transverse/sigmoid sinus acquired from dominant sinus occlusion by a tumor: report of two cases. Neurosurgery. 1998; Feb. 42(2):383–8.
2. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990; Jun. 72(6):839–50.

3. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995; Feb. 82(2):166–79.

4. Chandra RV, Leslie-Mazwi TM, Mehta BP, Yoo AJ, Rabinov JD, Pryor JC, et al. Transarterial onyx embolization of cranial dural arteriovenous fistulas: long-term follow-up. AJNR Am J Neuroradiol. 2014; Sep. 35(9):1793–7.

5. Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998; Sep. 65(3):308–16.

6. Deguchi J, Yamada M, Kobata H, Kuroiwa T. Regional cerebral blood flow after acetazolamide challenge in patients with dural arteriovenous fistula: simple way to evaluate intracranial venous hypertension. AJNR Am J Neuroradiol. 2005; May. 26(5):1101–6.
7. Fukai J, Terada T, Kuwata T, Hyotani G, Raimura M, Nakagawa M, et al. Transarterial intravenous coil embolization of dural arteriovenous fistula involving the superior sagittal sinus. Surg Neurol. 2001; Jun. 55(6):353–8.

8. Halbach VV, Higashida RT, Hieshima GB, Rosenblum M, Cahan L. Treatment of dural arteriovenous malformations involving the superior sagittal sinus. AJNR Am J Neuroradiol. 1988; Mar-Apr. 9(2):337–43.
9. Horinaka N, Nonaka Y, Nakayama T, Mori K, Wada R, Maeda M. Dural arteriovenous fistula of the transverse sinus with concomitant ipsilateral meningioma. Acta Neurochir (Wien). 2003; Jun. 145(6):501–4. discussion 504.

10. Houser OW, Baker HL Jr, Rhoton AL Jr, Okazaki H. Intracranial dural arteriovenous malformations. Radiology. 1972; Oct. 105(1):55–64.

11. Ilyas A, Chen CJ, Ding D, Buell TJ, Raper DMS, Lee CC, et al. Radiation-induced changes after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurgery. 2018; Sep. 83(3):365–76.

12. Kim B, Jeon P, Kim K, Kim S, Kim H, Byun HS, Jo KI. Predictive Factors for Response of Intracranial Dural Arteriovenous Fistulas to Transarterial Onyx Embolization: Angiographic Subgroup Analysis of Treatment Outcomes. World Neurosurg. 2016; Apr. 88:609–18.

13. Kurl S, Saari T, Vanninen R, Hernesniemi J. Dural arteriovenous fistulas of superior sagittal sinus: case report and review of literature. Surg Neurol. 1996; Mar. 45(3):250–5.

14. Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015; May. 122(3):421–9.

15. Narayanan S. Endovascular management of intracranial dural arteriovenous fistulas. Neurol Clin. 2010; Nov. 28(4):899–911.

16. Natarajan SK, Ghodke B, Kim LJ, Hallam DK, Britz GW, Sekhar LN. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010; Apr. 73(4):365–79.

17. Oh SH, Choi JH, Kim BS, Lee KS, Shin YS. Treatment outcomes according to various treatment modalities for intracranial dural arteriovenous fistulas in the Onyx era. A 10-year single-center experience. World Neurosurg. 2019; Jun. 126:e825–34.

18. Quigg M, Yen CP, Chatman M, Quigg AH, McNeill IT, Przybylowski CJ, et al. Risks of history of diabetes mellitus, hypertension, and other factors related to radiation-induced changes following Gamma Knife surgery for cerebral arteriovenous malformations. J Neurosurg. 2012; Dec. 117 Suppl:144–9.

19. Simon S, Yao T, Ulm AJ, Rosenbaum BP, Mericle RA. Dural arteriovenous fistulas masquerading as dural sinus thrombosis. J Neurosurg. 2009; Mar. 110(3):514–7.

20. Song W, Sun H, Liu J, Liu L, Liu J. Spontaneous resolution of venous aneurysms after transarterial embolization of a variant superior sagittal sinus dural arteriovenous fistula: case report and literature review. Neurologist. 2017; Sep. 22(5):186–95.
21. Vanlandingham M, Fox B, Hoit D, Elijovich L, Arthur AS. Endovascular treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 2014; Feb. 74 Suppl 1:S42–9.

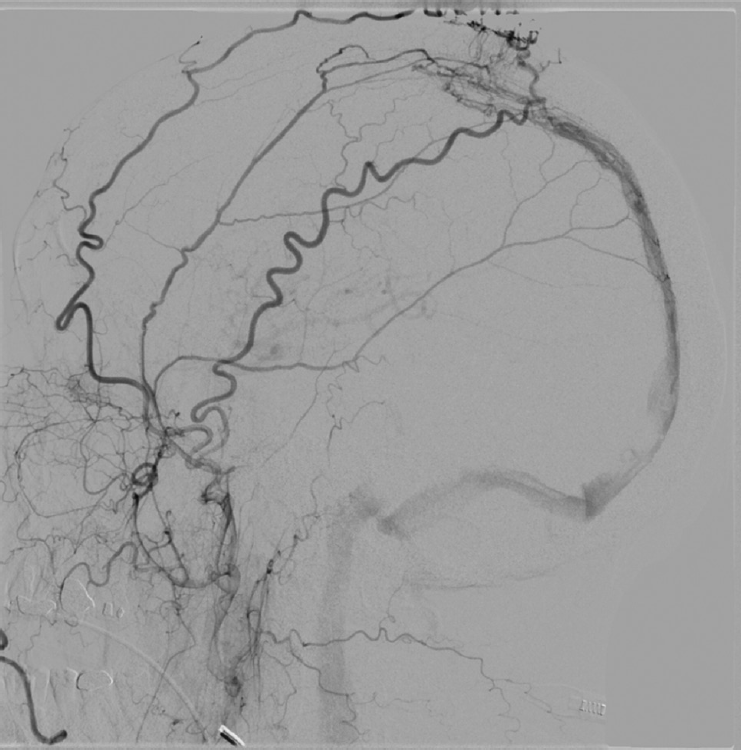
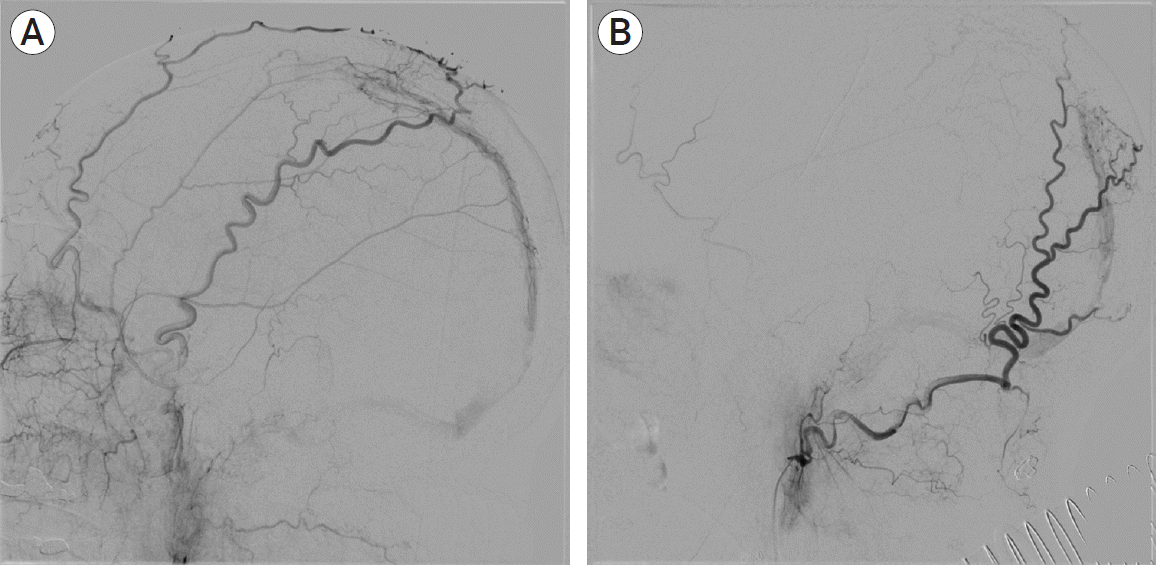
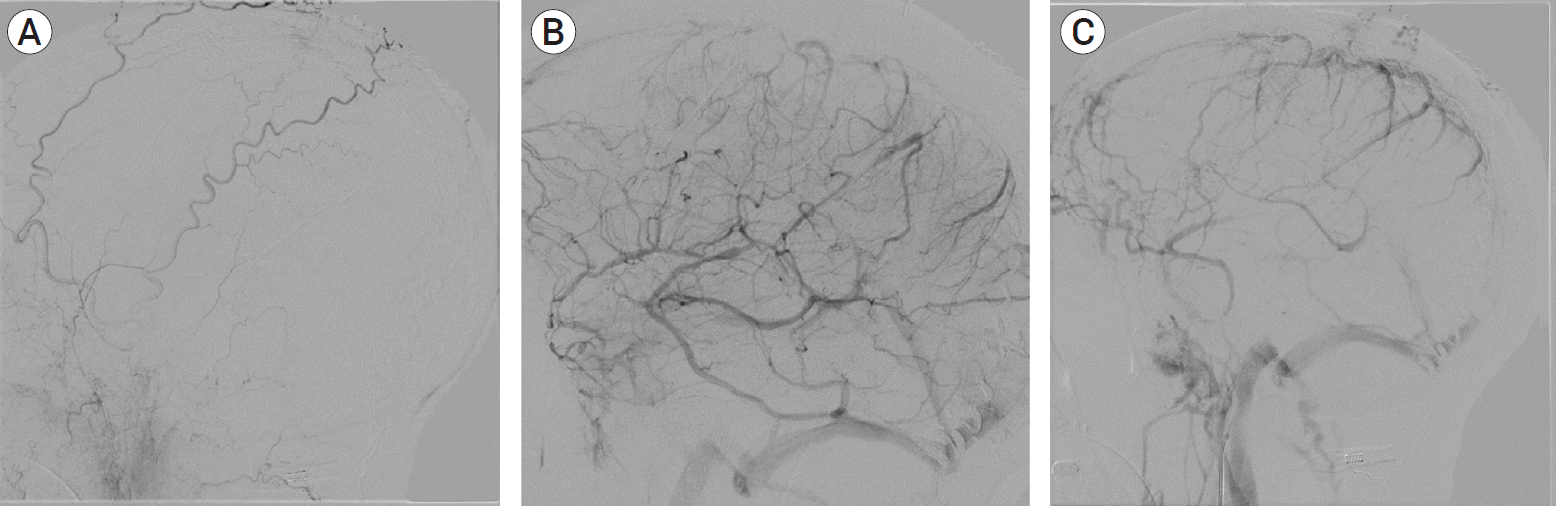




 PDF
PDF Citation
Citation Print
Print



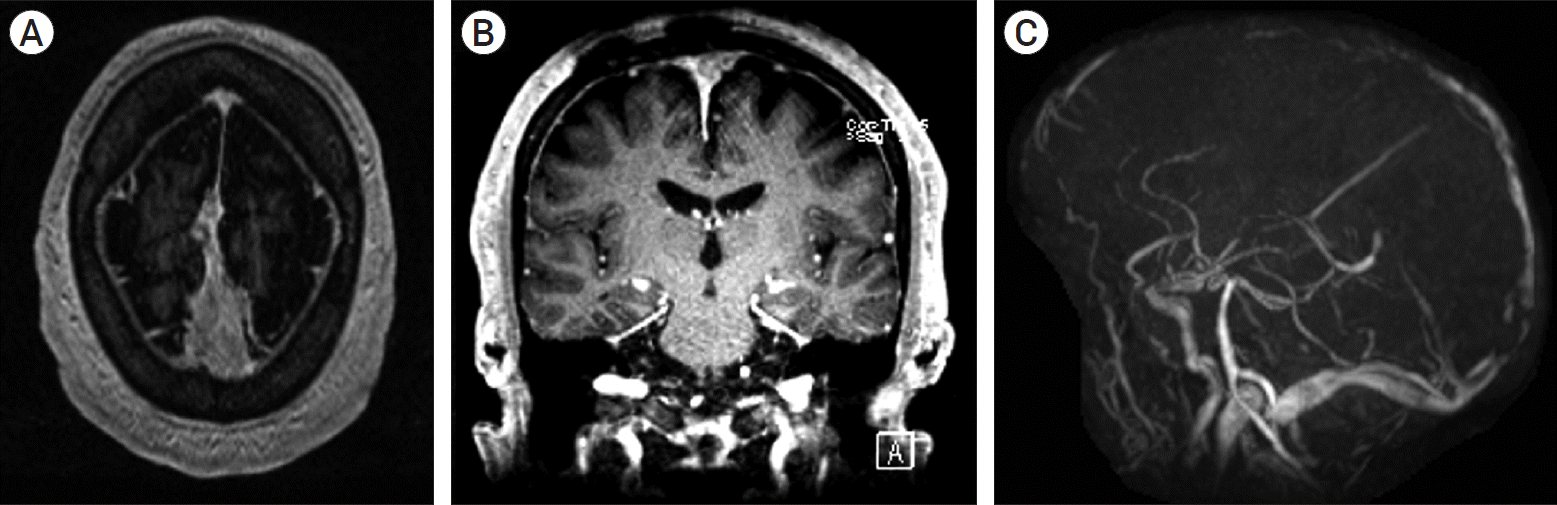
 XML Download
XML Download