Abstract
Spontaneous intracerebral hemorrhage (ICH) from a traumatic carotid-cavernous fistula (CCF) is a rare occurrence with few cases reported in the literature. Patients classically present shortly after the inciting trauma with symptoms of ocular venous hypertension. We report a case of an ICH due to delayed rupture of a venous aneurysm from a CCF in a patient with decades-old history of enucleation of the left globe secondary to trauma with no sentinel symptoms. Our patient represents a unique presentation of a rare pathology. This case highlights the need for ongoing surveillance in patients with a history of severe craniofacial trauma, as ICH from ruptured CCF(s) demands emergent treatment due to the potential for rapid neurological deterioration.
Carotid-cavernous fistula (CCF)s are rare vascular abnormalities that arise from a communication between the carotid artery and the cavernous sinus (CS). Assessment of CCFs takes into consideration the anatomy of involved vessels as well as the etiology, direction, and velocity of flow [17]. The majority of CCF(s) are traumatic in origin, although spontaneous and iatrogenic etiologies have been reported [1,2,13,15]. These lesions are often associated with high flow which is the basis for their acute presenting symptoms. Patients typically present with immediate symptoms of ocular hypertension, which include decreased visual acuity, ophthalmoplegia, chemosis, exophthalmos and cranial nerve deficits. Such rapid and drastic symptoms allow for early identification and treatment. Hemorrhagic presentation is seen in only 8.4% of CCFs and includes epistaxis, subarachnoid hemorrhage (SAH), and ICH [10]. ICH alone is seen in only 2.6% and is often preceded by ocular symptoms in the acute period of inciting trauma.
We report an interesting case of an ICH due to a delayed rupture of a venous aneurysm arising from a CCF in a patient with decades-old history of enucleation of the ipsilateral orbit secondary to trauma with no sentinel symptoms. We discuss the management of this patient and provide a review of the literature of all reported delayed traumatic CCF(s) presenting with spontaneous ICH.
A 54-year-old male with a past medical history of polysubstance abuse and a gunshot wound (GSW) to the left eye presented with sudden altered mentation and right-sided hemiparesis. Computed tomography (CT) imaging revealed a large left frontal lobe ICH with intraventricular hemorrhage (IVH). There were no reported ocular symptoms. Visual examination was significant for left eye enucleation, an intact pupillary light reflex and extraocular movements in the right eye. Visual acuity could not be assessed secondary to agitation. There was no evidence of exophthalmos in the right eye nor was there any evidence of chemosis.
Initial CT-angiogram (CTA) of the head and neck revealed decreased caliber of the left common carotid artery with concern for internal carotid artery (ICA) occlusion. There was evidence of significantly dilated vessels near the left CS and basal venous system and distended varices near the hemorrhage (Fig. 1). The patient’s lesion was thought to represent either a CCF or arteriovenous malformation. An emergent external ventricular drain (EVD) was placed and the patient was taken to the angiography suite.
Diagnostic cerebral angiogram confirmed chronic left ICA occlusion and the presence of a large, left-sided CCF. There was evidence of enlarged external carotid artery (ECA) branches which anastomosed with the distal ICA as well as multiple fistulous connections to the CS with occlusion of the cavernous ICA (Fig. 2). Right-sided ICA injections demonstrated an enlarged right ICA with filling across the anterior communicating artery complex to the left hemisphere and retrograde flow within the left ICA with a CCF and significant venous hypertension. There was evidence of reflux into the superficial and deep venous systems. This included the basal vein of Rosenthal, the middle cerebral vein and several frontal cortical veins. This resulted in venous ectasia and a large venous aneurysm of the thalamostriate vein in the white matter adjacent to the left frontal horn, which corresponded to the site of hemorrhage.
Upon diagnosis of the left CCF, transarterial embolization was performed using a transcirculation route via the anterior communicating artery. Two echelon (MicroTherapeutics, Irvine, CA, USA) microcatheters were navigated into the left supraclinoid ICA to the CS and into the origin of draining vein connecting to the CS. The vein was initially embolized with coils to optimize the delivery of the subsequent embolysate, Onyx (ethylene-vinyl alcohol copolymer (EVOH), ev3 Neurovascular, Irvine, CA, USA). This resulted in near-complete occlusion of the CCF with no further evidence of cortical venous reflux (Fig. 3).
Intraprocedurally, the patient experienced transient intracranial pressure (ICP) elevations which were responsive to medical therapy. Upon completion of the embolization, these elevations became refractory and a post-procedural CT scan demonstrated expansion of the hematoma and IVH (Fig. 4). The decision was made to perform an emergent decompressive hemicraniectomy and hematoma evacuation. Post-operatively, the patient did not have significant improvement in clinical examination. Given the patient’s persistent poor neurological examination over the next several days the family decided to withdraw care. The patient ultimately expired on hospital day 20.
A comprehensive literature search was conducted to identify all reports of traumatic CCF(s) that presented with ICH and/or IVH. We searched PubMed, Google Scholar, and Embase databases from inception to April 2020 to identify all pertinent journal articles. Key words included “carotid-cavernous sinus fistula”, “cerebral hemorrhage”, and “trauma”. Reference lists were examined to identify additional subjects. Other literature reviews were also evaluated.
Eligible studies included those in the English language for adult patients ≥18-year-old that reported ICH and/or IVH from a traumatic CCF. Traumatic causes encompassed both blunt and penetrating trauma including those in relation to craniofacial surgery. Studies reporting a CCF arising from a cavernous ICA aneurysm rupture or those failing to report an etiology of the CCF were excluded from analysis. In addition, cases presenting solely with SAH, epistaxis or venous infarction were excluded. Lastly, articles describing ICH as a complication of surgical treatment were excluded.
Our literature review yielded 15-reports totaling 16-patients (Table 1)[5-9,11,12,14,16,18-21,23,24]. Patients presented with ICH at an average age of 45 years (standard deviation=17 years), 50% of subjects were male, and the most common etiology of trauma was a motor vehicle accident (MVA). 75% (12/16) had symptoms prior to the ICH. The most common symptoms involved stigmata of ocular venous hypertension (92%, 11/12 cases). One case described a patient with facial nerve palsy and hemiparesis as a result of mass effect on the pons from a venous varix [5]. Time intervals between onset of symptoms and ICH were not consistently reported. Of studies that provided this information, the median interval was approximately 7 weeks. The majority of patients (63%, 10/16) required surgical evacuation of their ICH and/or cerebrospinal fluid diversion. 56% (9/16) of patients sustained a permanent neurological deficit, 6% (1/16) died, 13% (2/16) made a full recovery and the remaining 25% (4/16) of reports did not provide sufficient information pertaining to outcome.
Historically, CCF(s) were classified by their etiology (traumatic vs. spontaneous), hemodynamic properties (high flow vs. low flow), or anatomic characteristics. The Barrow classification groups CCF(s) into four objective angiographic categories based on arterial supply [4]. This classification is more relevant to the patient’s clinical presentation, prognosis, and treatment. Type A fistulas describe direct shunts between the cavernous ICA and the CS which are high-flow and almost never resolve spontaneously. In contrast, Type B, C and D fistulas are indirect shunts between the ICA, ECA and both respectively. These tend to be more insidious in onset, low-flow and sometimes can resolve without treatment.
With increasing understanding of the pathophysiology of CCF(s), the significance of venous outflow pathways became clear. Moreover, the importance of venous drainage patterns was highlighted as treatment paradigms shifted away from parent artery occlusion and transarterial detachable balloon embolization to more contemporary transvenous endovascular therapy using coils or liquid embolic agents. Venous outflow patterns in a CCF can be divided into five types: anteriorly into ophthalmic veins, inferiorly into the pterygoid plexus or inferior petrosal sinus, posteriorly into the deep venous system or superior petrosal sinus, superficially towards the superficial sylvian vein and contralaterally [3]. In the case of posterior drainage, as in our patient, arterial pressure is more easily transmitted to the cerebral veins. Previous reports have correlated the site of the ICH to that of venous engorgement [12,23]. Thomas et al. recently developed a venous-drainage based classification system which has been shown to correlate with symptomatology and treatment approach [22]. Whereas the Barrow classification system focuses on arterial supply, this newer system emphasizes how in current endovascular management, venous drainage patterns may have more significance than arterial ones.
The illustrated case presents a complex CCF that can be characterized as Type A & D. While the patient likely developed a characteristic Type A fistula secondary to his initial GSW, subsequent carotid occlusion masked any acute symptomatology at the time. Furthermore, enucleation eliminated anterior pathways of venous egress from the CS. On angiography, the ipsilateral supraclinoid ICA remained patent due to the low-resistance circuit via the anterior communicating artery complex. The left common carotid angiogram demonstrated ICA occlusion with the development of collateral arterial feeders from the ECA supplying the fistula. Moreover, extracranial-intracranial collateral circulation arising from the internal maxillary and ascending pharyngeal arteries likely contributed to the persistent filling of the distal ICA. The combination of these feeders resulted in a high-flow CCF as evidenced by multiple flow-related aneurysms and severe cortical venous hypertension. In the aforementioned venous classification scheme, our patient would fall into type IV which involves retrograde drainage into cortical veins. This type of CCF can be successfully treated by occlusion of the draining venous channel immediately distal to the fistulous point
The majority of patients develop a direct CCF secondary to trauma and most commonly present with symptoms related to ocular hypertension—the absence of ocular symptoms in a direct CCF is very rare. Due to the configuration of anastomoses to the CS, high pressure can be transmitted to cerebellar veins, temporal lobe veins, and the deep venous system. Venous hypertension can lead to venous ectasia or even ICH. Venous ectasia in turn can produce deficits from mass effect on critical structures [5]. We present a case of a patient with a history of a GSW to the left orbit and a delayed left-sided ICH with no sentinel symptoms. Enucleation led to masking of ocular symptoms, and undiagnosed venous hypertension manifested as ICH. Delayed ICH secondary to absence of ocular symptoms makes this a unique presentation.
Our literature review identified only 4 cases with no symptoms prior to ICH. The first patient had undergone a major maxillofacial surgery with iatrogenic trauma; however, significant facial swelling made assessment of symptoms difficult [12]. The second patient was involved in a MVA with facial trauma, also limiting assessment [11]. In the third case, D’Angelo et al described a 76-year-old female who sustained maxillofacial trauma after a MVA and suffered an ICH with no preceding ocular symptoms [8]. This was attributed to a low-flow venous drainage pattern although no posterior cortical venous outflow could be identified by the authors. The fourth case involved a Type A fistula treated with ICA ligation that later recanalized due to development of ECA collaterals [24].
The time between initial symptoms of a CCF and hemorrhage was reported to range between several days to 6 years with a median of 7 weeks in our review. However, in the illustrated case, a 38-year time span marked the interval between the inciting trauma and resultant ICH from a traumatic CCF. Workman et al described an interesting delayed presentation with a comparable timeline to ours: a 9-year-old male who sustained a GSW to the head, immediately developed a CCF, and underwent surgical ligation of the left ICA [24]. He later presented at the age of 35 with a cerebellar ICH. It was found that he had developed ECA collaterals, which re-constituted the cavernous ICA and re-formed the fistula over time. In a similar fashion, our patient appeared to have developed venous hypertension over a long period of time, explaining the occurrence of ICH after a long absence of symptoms. The delayed presentation in these cases highlights the need for a long-term follow-up in this patient population.
Venous hemorrhage from a CCF can progress rapidly. Half of the patients we identified required a surgical procedure and a majority suffered a permanent deficit or death. This highlights the importance of intracranial vascular surveillance in patients with traumatic maxillofacial and orbital history, particularly in those where symptoms of ocular hypertension may not be obvious. Endovascular occlusion of the CCF should ideally precede an open surgical evacuation of the ICH so as to avoid intraoperative venous hemorrhage [9,16].
In summary, we present a case of delayed ICH associated with a decades-old traumatic CCF, whose clinical evaluation was masked by orbital enucleation due to the original injury. This case is unique in that nearly 40 years had elapsed between the initial trauma and presentation of the CCF with spontaneous ICH, which is the longest interval reported in the literature. This case report and literature review highlights the urgent nature of this condition and the importance of intracranial vascular surveillance of select patients with orbital and maxillofacial injuries.
REFERENCES
1. Al-Mufti F, Amuluru K, El-Ghanem M, Changa A, Singh P, Gandhi C, et al. Spontaneous bilateral carotid-cavernous fistulas secondary to cavernous sinus thrombosis. Neurosurgery. 2017; Apr. 80(4):646–54.

2. Amuluru K, Al-Mufti F, Gandhi CD, Prestigiacomo CJ, Singh IP. Direct carotid-cavernous fistula: A complication of, and treatment with, flow diversion. Interv Neuroradiol. 2016; Oct. 22(5):569–76.

3. Aralasmak A, Karaali K, Cevikol C, Senol U, Sindel T, Toprak H, et al. Venous drainage patterns in carotid cavernous fistulas. ISRN Radiol. 2014; Jan. 2014:760267.

4. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985; Feb. 62(2):248–56.

5. Chan FH, Shen CY, Liu JT, Li CS. Brainstem hemorrhage caused by direct carotid-cavernous fistula. A case report and literature review. Interv Neuroradiol. 2014; Jul-Aug. 20(4):487–94.
6. Chang CM, Cheng CS. Late intracranial haemorrhage and subsequent carotid-cavernous sinus fistula after fracture of the facial bones. Br J Oral Maxillofac Surg. 2013; Dec. 51(8):e296–8.

7. Chavan RG, Kamble RB, Bonde V. Endovascular treatment in an unusual case of direct carotid cavernous fistula. Neuroradiol J. 2014; Apr. 27(2):207–12.

8. D’Angelo L, Paglia F, Caporlingua A, Sampirisi L, Guidetti G, Santoro A. Atypical manifestation of direct low-flow carotid-cavernous fistula: case report and review of the literature. World Neurosurg. 2019; May. 125:456–60.
9. d’Angelo VA, Monte V, Scialfa G, Fiumara E, Scotti G. Intracerebral venous hemorrhage in “high-risk” carotid-cavernous fistula. Surg Neurol. 1988; Nov. 30(5):387–90.
10. Halbach VV, Hieshima GB, Higashida RT, Reicher M. Carotid cavernous fistulae: indications for urgent treatment. AJR Am J Roentgenol. 1987; Sep. 149(3):587–93.

11. Hayashi K, Suyama K, Nagata I. Traumatic carotid cavernous fistula complicated with intracerebral hemorrhage: case report. Neurol Med Chir (Tokyo). 2011; 51(3):214–6.
12. Hiramatsu K, Utsumi S, Kyoi K, Sakaki T, Tada T, Iwasaki S, et al. Intracerebral hemorrhage in carotid-cavernous fistula. Neuroradiology. 1991; 33(1):67–9.

13. Imrie A, Redmond K, Leggett D. Spontaneous direct carotid-cavernous sinus fistula secondary to a persistent primitive trigeminal artery treated by trans-venous coil embolisation. Interv Neuroradiol. 2018; Oct. 24(5):567–70.

14. Kamio Y, Hiramatsu H, Kamiya M, Yamashita S, Namba H. Cerebellar hemorrhage due to a direct carotid-cavernous fistula after surgery for maxillary cancer. J Korean Neurosurg Soc. 2017; Jan. 60(1):89–93.

15. Lang M, Habboub G, Mullin JP, Rasmussen PA. A brief history of carotid-cavernous fistula. J Neurosurg. 2017; Jun. 126(6):1995–2001.

16. Lee CJ, Choi SW, Kim SH, Youm JY. Traumatic carotid-cavernous fistula bringing about intracerebral hemorrhage. J Korean Neurosurgc Soc. 2005; Aug. 38(2):155–7.
17. Leone G, Renieri L, Enriquez-Marulanda A, Dmytriw AA, Nappini S, Laiso A, et al. Carotid cavernous fistulas and dural arteriovenous fistulas of the cavernous sinus: Validation of a new classification according to venous drainage. World Neurosurg. 2019; Aug. 128:e621–31.

18. Lin TK, Chang CN, Wai YY. Spontaneous intracerebral hematoma from occult carotid-cavernous fistula during pregnancy and puerperium. Case report. J Neurosurg. 1992; Apr. 76(4):714–7.
19. Moon KY, Kang SD. Spontaneous intracerebral hematoma from transient occult carotid-cavernous fistula: a case report. J Korean Med Sci. 2005; Feb. 20(1):166–8.
20. Nagesh CP, Mohimen A, Kannath SK, Rajan JE. Primary intraventricular haemorrhage due to rupture of giant varix of the basal vein of Rosenthal in a patient with long-standing direct CCF: angiographic features and treatment considerations. BMJ Case Rep. 2017; Nov. 2017:bcr2017013396.

21. Tanaka A, Fukushima T, Tomonaga M. Intracerebral hematomas in cases of dural arteriovenous malformation and carotid-cavernous fistula. Surg Neurol. 1986; Jun. 25(6):557–62.

22. Thomas AJ, Chua M, Fusco M, Ogilvy C, Tubbs RS, Harrigan M, et al. Proposal of venous drainage – based classification system for carotid cavernous fistulae with validity assessment in a multicenter cohort. Neurosurgery. 2015; Sep. 77(3):380–5.
23. Turner DM, Vangilder JC, Mojtahedi S, Pierson EW. Spontaneous intracerebral hematoma in carotid-cavernous fistula. Report of three cases. J Neurosurg. 1983; Oct. 59(4):680–6.
24. Workman MJ, Dion JE, Tong FC, Cloft HJ. Treatment of Trapped CCF by Direct Puncture of the Cavernous Sinus by Infraocular Trans-SOF Approach. Case Report and Anatomical Basis. Interv Neuroradiol. 2002; Sep. 8(3):299–304.
Fig. 1.
Axial CTA reveals cavernous sinus venous sacs and a dilated deep venous system (A). A venous network in the temporal lobe with connections to superficial cortical veins (B). Axial and coronal images show a periventricular venous varix arising from the thalamostriate vein (C, D). CTA, computed tomography angiogram.
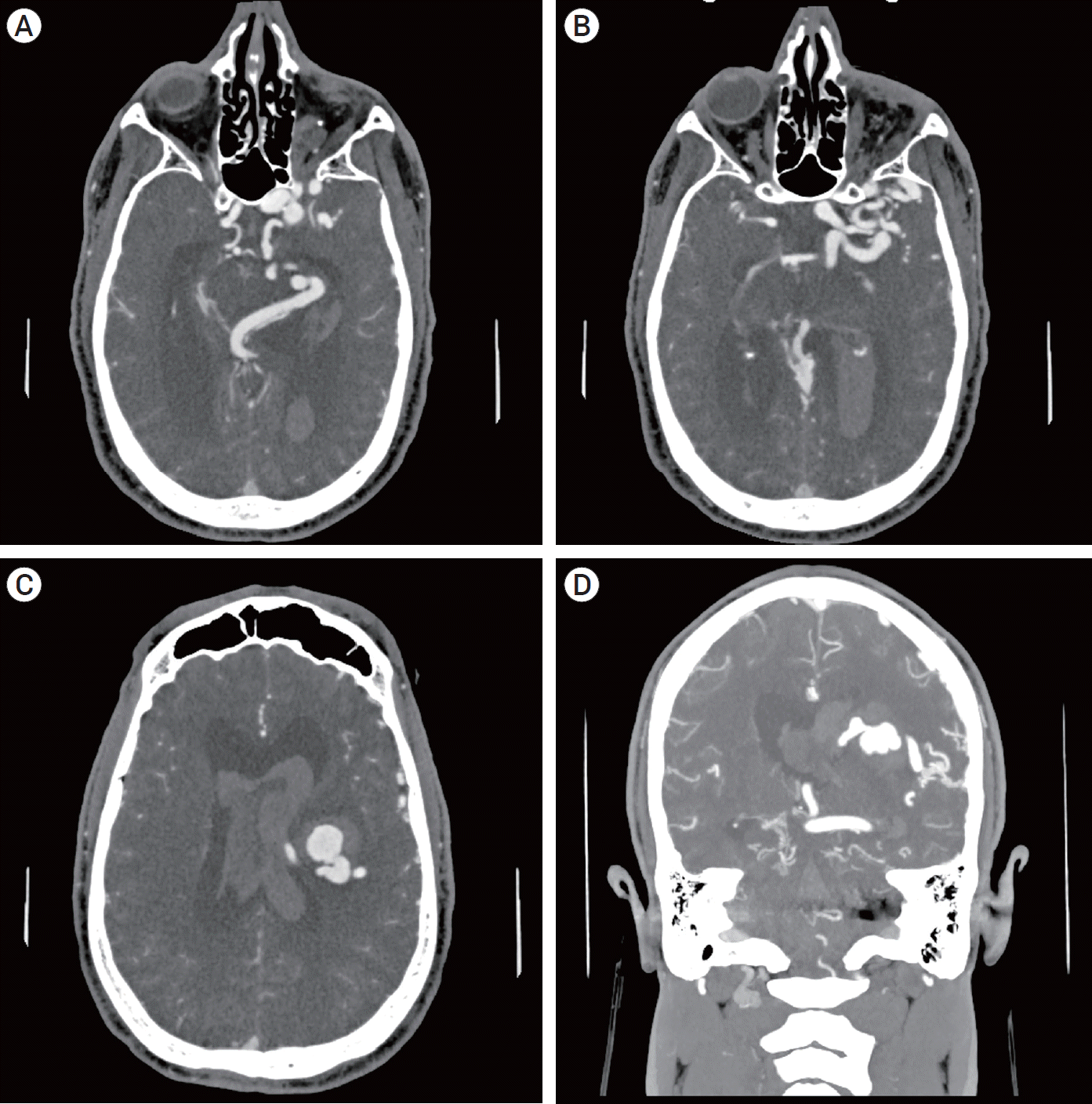
Fig. 2.
AP DSA of the right ICA in the arterial phase shows rapid cross-flow through the anterior communicating artery complex into the CCF (A). There is evidence of venous hypertension as manifested by the dilation of both deep and superficial venous systems (B, C). Lateral DSA of the left CCA shows proximal ICA occlusion with supply of the CCF by way of ICA and ECA feeders (D). Oblique DSA of the right ICA and AP DSA of the right vertebral artery show flow-related aneurysms in the right A2 segment of the anterior cerebral artery and the basilar apex (E, F). AP, anteroposterior; DSA, digital subtraction angiography; ICA, internal carotid artery; CCF, carotid-cavernous fistula; CCA, common carotid artery; ECA, external carotid artery.
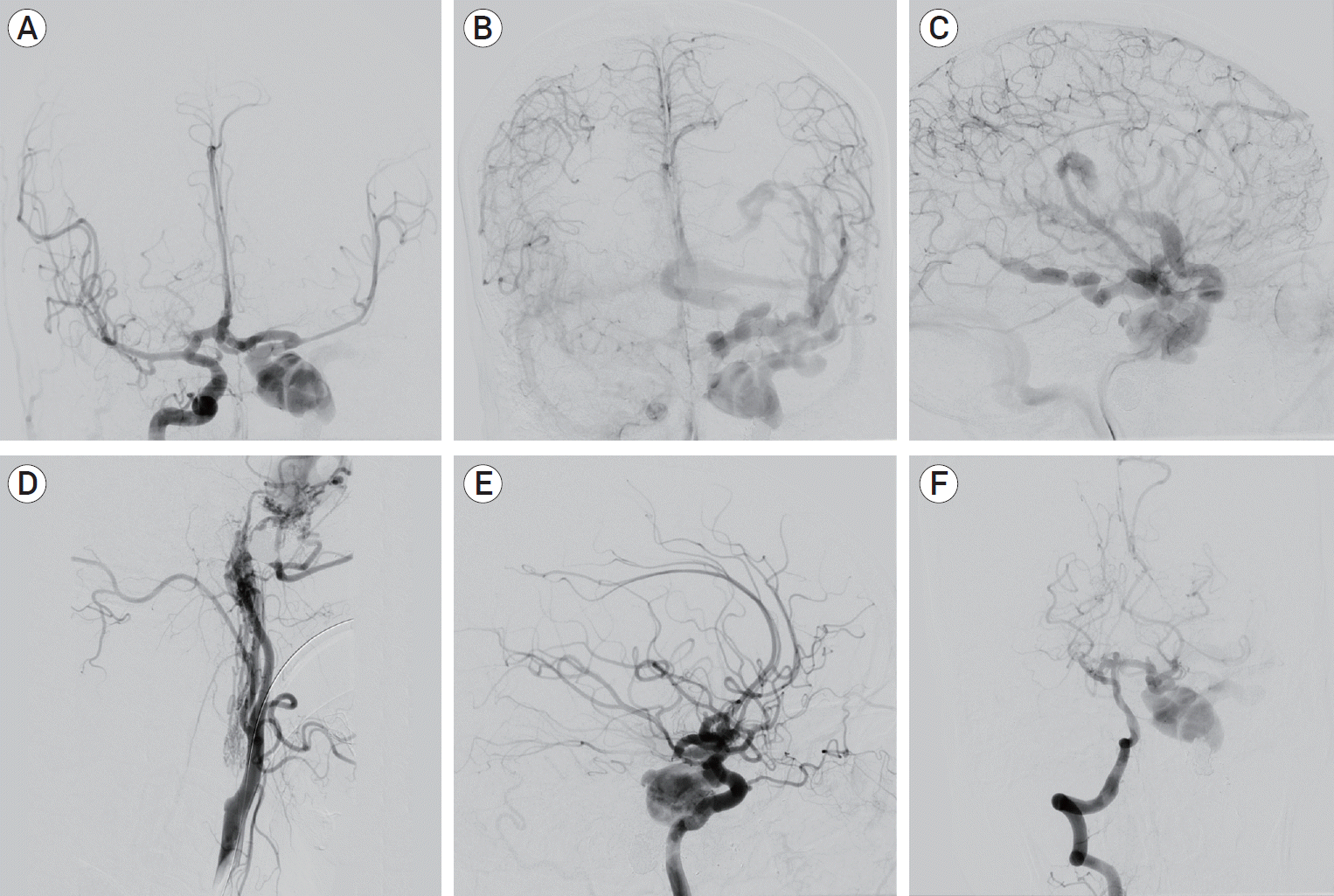
Fig. 3.
Oblique unsubtracted view shows the combined Onyx-Coil mass (A). AP and lateral DSA of the right ICA after embolization shows near-complete occlusion of the CCF with no further evidence of retrograde venous reflux. The ACA territory fills from the right ICA bilaterally and there is faint filling of the left MCA territory from the right ICA injection (B, C). AP DSA of the left vertebral artery after embolization demonstrates filling of the left MCA territory by the posterior circulation via the posterior communicating artery (D). AP, anteroposterior; DSA, digital subtraction angiography; ICA, internal carotid artery; CCF, carotid-cavernous fistula; ACA, anterior cerebral artery; MCA, middle cerebral artery.
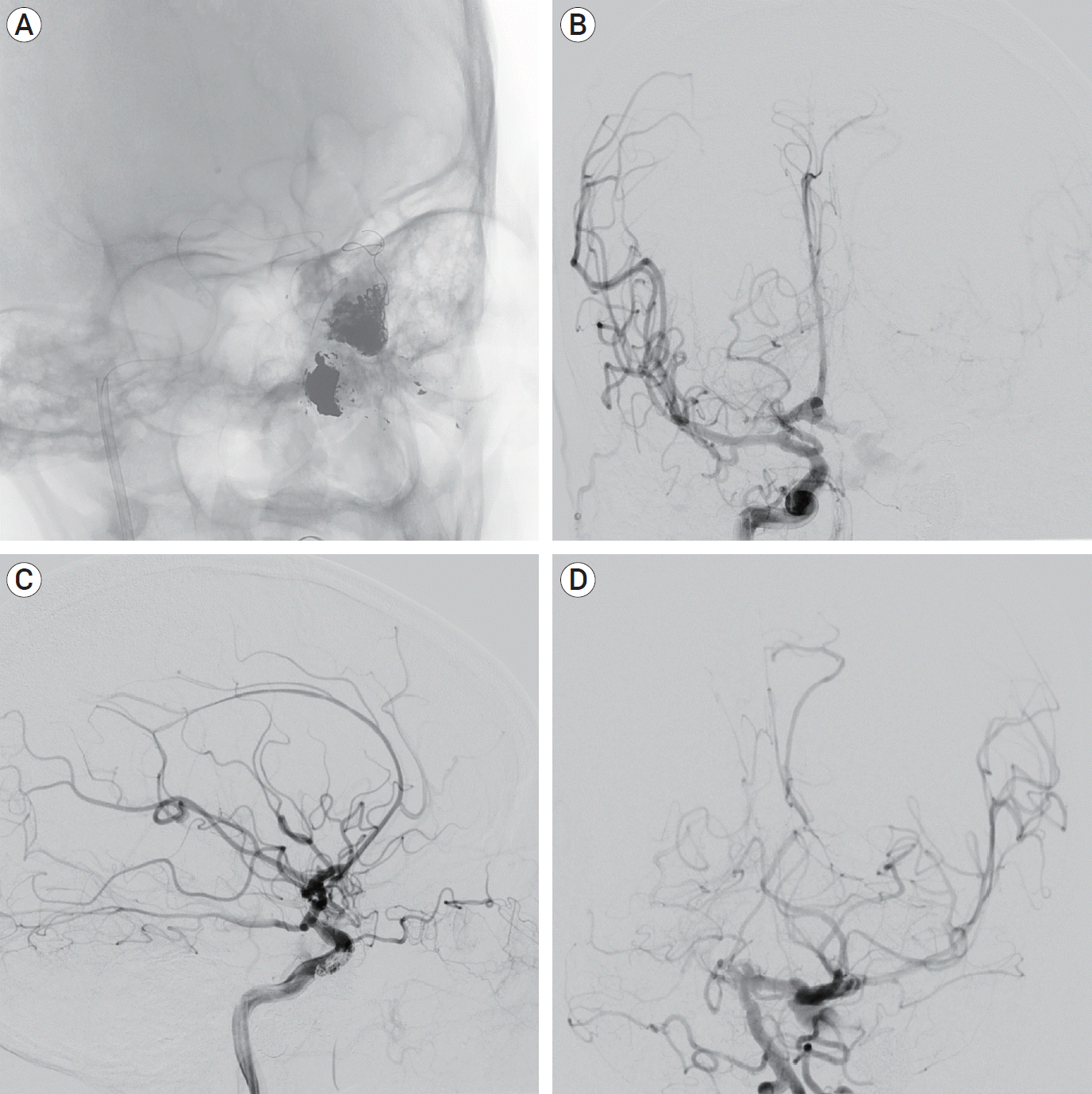
Fig. 4.
Post-procedural axial computed tomography (CT) scan shows evidence of an expanding hematoma, IVH and midline shift (A). Post-operative axial CT scan after decompressive hemicraniectomy for hematoma evacuation (B). IVH, intraventricular hemorrhage.
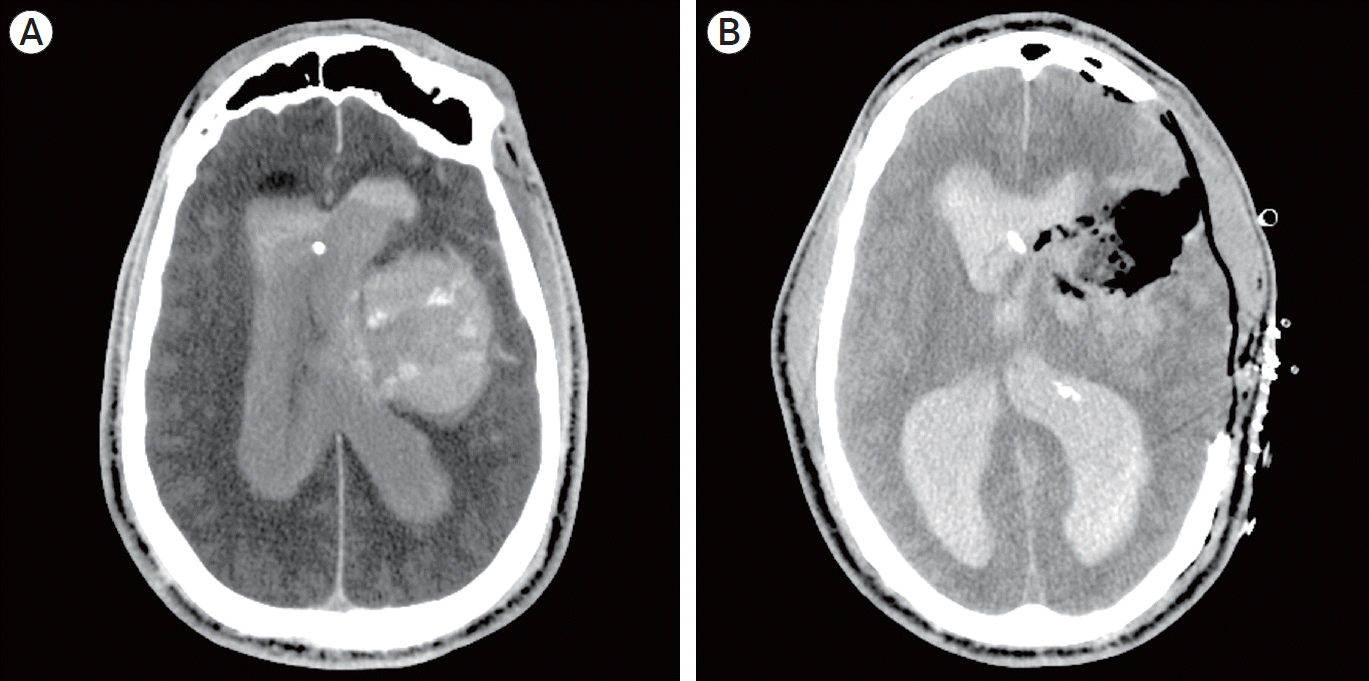
Table 1.
Clinical data of published traumatic CCF(s) presenting with ICH and/or IVH
| Case No. | Authors & Year | Age (yrs), sex | Hemorrhage location | Trauma etiology | Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1&2 | Turner et al. [23], (1983) | #1-68, M | #1-R Frontal, Intraventricular | #1-Fall | #1-Ocular sx preceding ICH | #1-EVD, Crani ICH evac, packing of CCF | #1-Permanent neurological deficit |
| #2-54, M | #2-L Frontotemporal | #2-MVA | #2-Ocular sx preceding ICH with re-hemorrhage | #2-DBE, ICA ligation | #2-Permanent neurological deficit | ||
| 3 | Tanaka et al. [21], (1986) | 49, F | R Frontal | Fall | Ocular sx preceding ICH | Crani ICH evac, ICA ligation | Permanent neurological deficit |
| 4 | d'Angelo et al. [9], (1988) | 41, F | L Temporal | Not reported | Ocular sx preceding ICH | DBE, Crani ICH evac | Permanent neurological deficit |
| 5 | Hiramatsu et al. [12], (1990) | 62, F | L Frontoparietal | MVA | Ocular sx preceding ICH | DBE, Crani ICH evac | Deceased |
| 6 | Lin et al. [18], (1992) | 30, F | R Frontotemporal | MVA | Ocular sx preceding ICH. Two subsequent re-hemorrhages | Crani ICH evac X 3. Trapping & muscle embolization of ICA | Permanent neurological deficit |
| 7 | Workman et al. [24], (2002) | 35, M | L Cerebellar | GSW | No sx preceding ICH | Trapping & ligation of ICA at age 9 DP | Full recovery |
| 8 | Lee et al. [16], (2005) | 26, M | R Frontal, Intraventricular | MVA | Ocular sx preceding ICH | DBE, burr-hole ICH evac | Insufficient information to determine* |
| 9 | Moon and Kang [19] (2005) | 60, M | L Frontal | Fall | Ocular sx preceding ICH | Crani ICH evac, TAE | Not reported |
| 10 | Hayashi et al. [11], (2011) | 45, M | L Frontal, Intraventricular | MVA | ICH preceding ocular sx | TVE, attempted TAE, ICA occlusion | Permanent neurological deficit |
| 11 | Chang and Cheng [6] (2013) | 27, F | R Pontine | MVA | Ocular sx preceding ICH | EVD, TAE | Not reported |
| 12 | Chavan et al. [7], (2014) | 25, M | R Temporal | MVA | Ocular sx preceding ICH | Crani ICH evac, TAE, Repeat TAE | Full recovery |
| 13 | Chan et al., [5] (2014) | 34, F | L Pontine, Intraventricular | MVA | Symptoms from mass effect preceding ICH | EVD, TAE, ICA occlusion | Permanent neurological deficit |
| 14 | Kamio et al. [14], (2017) | 63, F | L Cerebellar | Maxillofacial surgery | Simultaneous ocular sx & ICH | TAE, TVE, ICA occlusion | Permanent neurological deficit |
| 15 | Nagesh et al. [20], (2017) | 28, M | Intraventricular | Not reported | Ocular sx preceding ICH | TAE, ICA occlusion | Insufficient information to determine* |
| 16 | D'Angelo et al. [8], (2019) | 76†, F | R Frontotemporal | Fall | ICH preceding ocular sx | Partial TVE | Permanent neurological deficit |
† A discrepancy was present between the age in this publication. The age reported in the abstract was listed here
Burr-hole ICH evac, Burr hole for evacuation of ICH; Crani ICH evac, Craniotomy for evacuation of ICH; IVH, intraventricular hemorrhage; DBE, detachable balloon embolization; DP, direct puncture; EVD, external ventricular drain; GSW, gunshot wound; ICH, intracerebral hemorrhage; MVA, motor vehicle accident; Sx, Symptoms; TAE, transarterial embolization; TVE, transvenous embolization; ICA, internal carotid artery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download