Abstract
Objective
Indocyanine green video angiography (ICG-VA) is a routine while performing vascular surgery to assess patency of perforators, completeness of clipping and/or to assess patency of anastomosis. Its usefulness in assessing cerebral blood flow and perfusion is not well studied. This study is aimed to assess the cerebral blood flow and perfusion after temporary clipping and to correlate with the risk of ischemia.
Methods
Prospective analysis of intra-operative ICG-VA performed during temporary arterial occlusion in 38 patients from January 2014 to December 2018 was conducted. Co-relation with post-operative MR diffusion weighted imaging (MR DWI) in terms of vascular territory of interest within 48 hours of surgery was performed. Clinical outcome was assessed using modified Rankin Scale (mRS) score 1-month post-surgery.
Results
43 aneurysms in 38 patients clipped using ICG-VA were included in this study. No side effect of ICG dye was seen in any patients. The number of times temporary clips applied had a direct relationship to the delay in appearance of ICG in the surgical field which became statistically significant after application of 3rd temporary clip. Nine (23.7%) patients developed ischemia following the procedure confirmed by post-operative MR DWI and all the ischemic cases had visible decrease in ICG fluorescence post-temporary clipping.
Temporary arterial occlusion (TAO) of vessels during surgical clipping of intracranial aneurysm is an established method that helps in dissection of aneurysms, controlling intra-operative aneurysm rupture (IAR) and helps the surgeon to place a permanent clip precisely at the neck of an aneurysm. However, this may lead to stroke due to impaired cerebral perfusion secondary to temporary occlusion of the feeding arteries [1,21]. Studies suggest that temporary clipping of the major feeding vessels should be broken up by sufficient recirculation time of at least 5 to 10 min when longer periods of arterial occlusion are necessary because ischemic brain damage depends mainly upon the duration of the vessel occlusion [14,19]. On the other hand, experimental studies have documented that repetitive episodes of brief global ischemia may cause cumulative detrimental effects in the form of ischemic neuronal damage, whereas these problems are not seen with a single ischemic episode of the same total duration [22]. These observations are a call to assess cerebral blood flow and perfusion while applying temporary clips to the feeding arteries. There are many direct or indirect ways of assessing cerebral blood flow and perfusion such as intra-operative digital subtraction angiography (DSA), doppler, positron emission tomography (PET), magnetic resonance (MR) perfusion studies, intra-operative and postoperative MR imaging (MRI) with diffusion weighted imaging and somatosensory evoked potential (SSEP). Most of these imaging procedures are technically demanding, expensive and requires additional time and human resources. Intra-operative Doppler, though frequently used, may not provide information regarding flow in the very small vessels as their ability to assess small perforating arteries is poor [4].
Intra-operative Indocyanine green video-angiography (ICG-VA) is a real-time evaluation of intra-operative visualization of parent vessels, perforators and aneurysms, and has an established role to assess the adequacy of clipping of cerebral aneurysms [5]. This study was conducted to study the cerebral blood flow during temporary clipping of aneurysm using ICG-VA and to assess its efficacy.
This study consisted of 122 prospectively enrolled ruptured aneurysm patients who underwent clip ligation during January 2014 to December 2018 using ICG-VA at our institute. Of them, temporary arterial occlusion was utilized in 38 patients with 43 aneurysms. Adult patients of either sex with intracranial aneurysms considered for surgery were included in the study and patients having history of iodine allergy, pregnancy or previous anaphylactic reactions to contrast media or dye injections were excluded. Elective temporary clips were utilized in all cases where neck was found to be calcified or dome contained thrombus to prevent embolic infarct and avoided in cases with atheromatous parent artery. All patients underwent standard microsurgical procedures for clip ligation of their aneurysms assisted by ICG-VA. The Institutional Ethics Committee reviewed and approved this study, and all patients provided consent before participation.
We used the NIR fluorescence module that was integrated into the surgical microscope (Moller Wedel, Germany) to assess the fluorescent areas in the operating field and generate a video of the emitted fluorescent light. Technical principles of ICG as described by Raabe et al. [16] was followed in every patient. The patients received 25 mg intravenous dose of ICG dissolved in 5 mL of 0.9% saline and given as a bolus of 1.5 mL per injection via cephalic vein after doing sensitivity test. Temporary clips were applied to assist dissection and ICG shoots were taken both before and after the temporary clip application to assess the status of branching and perforator arteries. In cases where parent or branching vessel stenosis or residual aneurysm necks were identified with ICG, the clip position was changed or additional clips were applied and another ICG-VA study was performed. Visual assessment of a real time play back of the procedure was performed in every case to note the appearance time of ICG in surgical field. Intra-operative monitoring of systolic blood pressure (SBP), heart rate (HR), pH, hematocrit (Hct), paCO2 and paO2 was done throughout the procedure.
MRI brain was performed within 48 hrs in 29 patients before and after the procedure to assess the development of ischemic areas. In poor grade patients, MRI was excluded. Ischemic change from inadvertent vessel occlusion during aneurysm clipping was avoided by assessing ICG-VA post placement of permanent clip and clip was re-adjusted whenever required. Patients were assessed in the postoperative period on the basis of radiological and clinical findings. Radiologic ischemia was defined as having ischemic changes on imaging but no neurologic deficit clinically and symptomatic ischemia was defined as having neurologic deficit as well as ischemic changes on radiologic imaging. Clinical outcome was assessed using modified Rankin Scale (mRS) score 1-month post-surgery. A good outcome was defined as mRS score of 0-2 while poor outcome was defined as mRS score of 3-6. The end points included the occurrence of radiologically documented ischemia on postoperative MRI scans, the development of symptomatic or asymptomatic stroke, and the clinical outcome.
Statistical analyses were done using SPSS 17.0 for windows (SPSS Inc., Chicago, IL, USA) and a p value of less than 0.05 was considered significant. Categorical and continuous variables were compared using the chi-square or Fisher tests and t-test when appropriate. Multivariate logistic regression models were used to analyze factors responsible for ischemic complications and outcome. Relative risk of the variables was obtained by calculating adjusted odds ratios and 95% confidence intervals.
Of the 38 patients who underwent temporary clipping, 25 were female and 13 were male ranging in age from 21 to 72 years (mean 46 years). Of them, 8 had serious systemic disease (hypertension, diabetes, or chronic lung disease). 29 patients presented in good clinical grade (World Federation of Neurosurgical Societies [WFNS] I-III), while 9 in poor grade (WFNS IV-V). The numbers of patients in admission CT Fisher grade 1, 2, 3 and 4 were 2, 28, 4 and 4 respectively. Of the 43 aneurysms, 20 (46.5%) were in anterior communicating artery (Acom); 10 (23.3%) in middle cerebral artery (MCA); 9 (20.9%) in anterior cerebral artery (including 8 distal anterior cerebral artery aneurysms), 3 (7%) in internal carotid artery- posterior communicating artery (ICA-Pcom) junction and 1 (2.3%) were in vertebrobasilar system. Of the 38 patients, 5 had coexistent unruptured aneurysms. All patients were operated within 48-hrs of admission and the mean surgical timing was 4 days from ictus.
During surgery, temporary clipping was utilized in 38 patients. Of the 43 aneurysms, 12 had temporary clipping once while 31 had repeated temporary clipping; 14 aneurysms had 2 times, 8 aneurysms had 3 times, 6 aneurysms had 4 times while 3 aneurysms had 5 times of repeated temporary clipping. Table 1 depicts the duration of application of temporary clips and Table 2 depicts the time interval between the application of temporary clips in seconds.
No side effects of ICG dye in any patient was observed in this study. Average time of appearance of ICG in surgical field (1st arterial phase) after administration of ICG via the cephalic vein was 22 secs (range 16-42 sec). ICGVA revealed either decreased number of visible perforators or diminished fluorescence post-removal of the clips in 9 patients (Fig. 1, 2 and 3).
Average delay time was calculated as the mean of delay time after each clip placement in all patients. The number of times temporary clips applied had a direct relationship to the time of appearance of ICG in the surgical field. The average delay was 0.56 secs after the application of 1st clip which increased to 2.16 secs after the 5th clip application (Table 3). The delay in appearance of ICG was statistically significant (p=≤0.05) after application of 3rd clip.
The blood gases, pH and hematocrit remained stable without any significant variation after application of temporary clips. Temporary clipping was associated with an immediate rise of SBP and this elevation lasted till the time temporary clip is in place and then gradually fell to baseline after the removal of clips. The elevation in SBP was more prominent as the number of times of clip applications increased (resting 115±18 mm Hg; after 5th clip 126±4 mm Hg) (Table 4).
Nine (23.7%) patients developed ischemia following the procedure evident on postoperative MRI scans as ischemic infarct related to the areas supplied by the perforating arteries. Of them, 6 (15.8%) patients (S. No.1-6; Table 5) developed symptomatic while 3 (7.9%) patients (S. No. 7-9; Table 5) developed asymptomatic ischemia. All the 9 patients had visible decrease in fluorescence and delay in appearance of ICG post temporary clipping. The number of perforators were decreased in 5 patients which could be assessed by ICG-VA.
There was no significant increase in the risk of ischemic complication in the patients 50 years of age or older in this study. Age younger than 50 years was associated with a 14% risk of symptomatic ischemia, while age 50 years or older was associated with a 15.6% risk of ischemia (univariate analysis, p=0.821).
The Hunt and Hess and Fisher grade of hemorrhage were related to the overall patient outcome but not to the risk of ischemic complication. A favorable outcome was achieved in 66.3% of the patients with poor grade hemorrhages (Hunt and Hess grade 4-5 and Fisher grade 3-4). Univariate analysis revealed that grade of hemorrhage was significantly associated with outcome as well as asymptomatic ischemia (p=≤0.001), but not with symptomatic ischemia.
Aneurysm characteristics like location and size larger than 10 mm were not significantly associated with a poor outcome except in cases of distal anterior cerebral artery (DACA) aneurysms. It was found that the incidence of symptomatic and asymptomatic ischemia was relatively high in patients with DACA which was statistically significant (p=0.003), however the outcome was not (p=0.24 and 0.526 respectively). The size of the aneurysm was not significantly associated with outcome (p=0.54), symptomatic (p=0.529) or asymptomatic ischemia (p=0.362).
One important factor that was associated with outcome was timing of surgery following subarachnoid hemorrhage (SAH); with patients undergoing surgery 96 hours after SAH were doing better than patients undergoing early surgery (p=0.020). Likewise, the rate of symptomatic ischemia was also higher in patients undergoing early surgery (p=0.001). Besides that, presence of cerebral edema (p<0.001) intraoperatively and need for ventricular drainage (p=0.002) were two factors that was significantly associated with symptomatic ischemia.
Post-temporary clip ICG-VA study revealed that at about 2 min 10 sec duration of temporary clip application, flow starts decreasing in the vessel and perforators distal to occlusion. Multiple application of temporary clip further hampers the flow distal to it. Univariate analysis found it to be statistically significant (p=0.005).
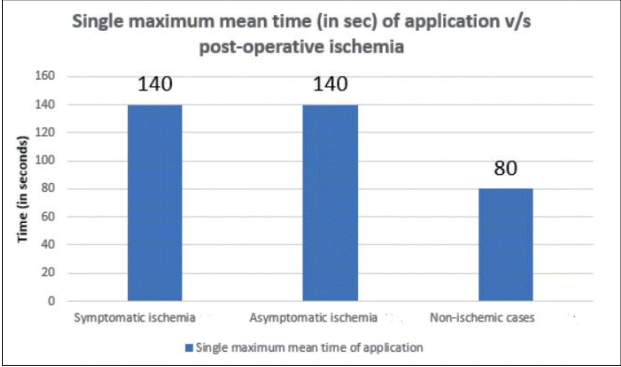
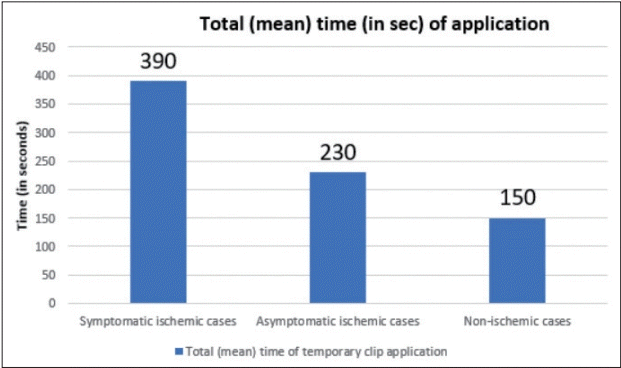
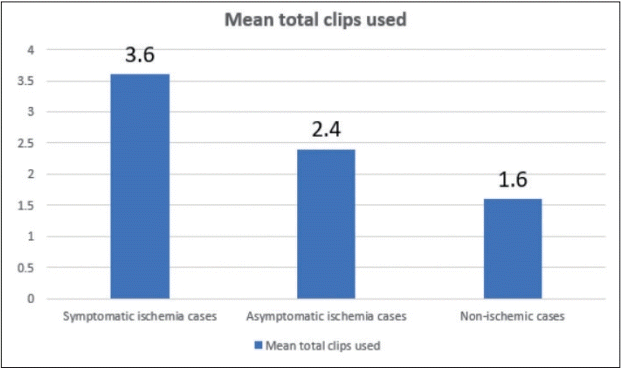
Single maximum mean time of the temporary clip used analysis revealed a mean time of 2 min 20 seconds in both symptomatic and asymptomatic ischemia (Fig. 4) while total (mean) time of application in symptomatic cases was 6 min 30 secs and 3 min 50 secs in asymptomatic cases (Fig. 5). The average number of temporary clips utilized was 3.6 in symptomatic and 2.4 in asymptomatic ischemia cases (Fig. 6).
The incidence of symptomatic ischemia was 16% (1 case) in patients undergoing temporary occlusion for periods of less than 5 minutes and 84% (5 cases) in patients undergoing temporary occlusion for more than 5 minutes although no association was seen between duration of occlusion and outcome (p=0.531). However, incidence of symptomatic ischemia was found to be directly related to duration of occlusion on univariate analysis (p=0.005).
On multivariate logistic regression analysis, independent factors predictive for ischemic complications and neurological outcome included preoperative vasospasm (odds ratio [OR] 1.2; confidence interval [CI] 1.02-3.02; p=0.002), mean number of temporary clips applied (OR 2.3; CI 1.36-3.4; p=0.05), mean total time of TAO (OR 2.86; CI 1.26-5.36; p=0.003) and WFNS grade <4 (OR 1.01; CI 0.98-1.06; p=0.035).
The mean follow-up time was 8 months (range, 3 months-16 months). A good outcome (mRS 0-2) was achieved in 32 (84.2%) patients, and 6 (15.8%) patients had poor outcomes (mRS 3-6). Analysis of mRS at 1-month post-surgery suggested average score of 2 in post clipping symptomatic infarct cases. It was 1 in asymptomatic infarct cases and 0 in non-ischemic cases.
The role of temporary occlusion to control IAR and to facilitate dissection has been well accepted and is used with increasing frequency in the management of intracranial aneurysms. Jefferson was the first to use elective temporary occlusion in 1928 for the treatment of intracranial aneurysms [15]. As the use of TAO during aneurysm surgery is gaining wider acceptance in the neurosurgical practice [2,3,6], it is prudent to define the risks of this procedure as well as to study the hemodynamic changes during its application and to identify a reasonably safe period of occlusion. Ischemia during TAO is difficult to predict and this is where the ICG-VA may play a vital role that may warn the surgeon in the form of diminished fluorescence, delay in appearance or decreased visibility of perforators intraoperatively.
We followed uniform anesthesia regimen in this study with of induction of normothermia and normotension in all cases with initiation of barbiturate infusion before aneurysm clipping in majority of cases. Some studies suggest hypertension or hypothermia during temporary occlusion, while others suggested use of different monitoring techniques to warn the surgeon of impending stroke [13,20]. However, these monitoring techniques remain technically challenging, imperfect and can be misleading [21] and the current study shows that simple assessment of vessel status before and after application of temporary clips is highly useful in predicting impending ischemia.
In our study, the SBP was found to be increased during TAO that remained throughout the application and returned to baseline slowly after its removal. This perhaps may be due to activation of cerebral autoregulation mechanism to maintain flow in vessels distal to occlusion.
Previous studies suggested association of older age with higher risk of stroke and poor outcomes which could probably be afflicted to associated medical conditions like hypertension and vascular diseases seen in elderly leading to greater risk of borderline cerebral perfusion [6,10]. However, current study could not detect any such association. We believe that shorter duration of temporary clips as well as keeping the number of TAO to a minimum possible number has resulted in better outcome in our series. However, more studies and greater number of patients are required to conclusively draw this finding.
Location was not a significant factor for ischemia, but the incidence of symptomatic ischemia was relatively high in patients with DACA aneurysms. We assume it to be due to its technically challenging location and longer duration of dissection requiring more numbers of TAO coupled with minimal cisternal CSF drainage and longer periods of brain retraction.
Timing of surgery is also an important variable for the development of ischemia and thus the outcome. In the acute stages, retraction of the brain parenchyma may lead to reduced perfusion pressure at the local site due to less tolerance which may lead to vasospasm and cerebral dysfunction of immediate or delayed onset [9,18]. Moreover, immediately following SAH, diminished cerebral perfusion develops independent of vasospasm, which also predisposes the patients to stroke. However, the benefits of delayed over early surgical intervention in regard to resistance to ischemia is counteracted by the risks of a wait and watch approach, especially when the overall management of patients with SAH ruptured aneurysms is considered which has also been confirmed by another study [7].
The duration of temporary occlusion remains the most investigated factor in clinical series of patients who undergo TAO [11,13]. In our study, we observed a trend for a decreased incidence of ischemic complication and good outcome in patients in whom temporary vessel occlusion was performed for less than 5 minutes. Furthermore, in our study, patients with repeated TAO, showed a higher incidence of stroke than those with single TAO. There are discordant reports regarding the mode of temporary occlusion [13]. Some authors have claimed that there is a higher risk of ischemia with intermittent occlusion based on the results of experimental studies, whereas others have claimed that the risk is higher when continuous occlusion is performed [11]. The temporary occlusion times reported in the studies in which the rate of ischemia was lower when continuous occlusion was performed and compared with intermittent occlusion also involved substantially longer occlusion times [13]. Previous studies [8,13] have suggested a wide range of safe occlusion time with one recent study [17] suggesting that duration of occlusion of 5 to 7 minutes can be safely tolerated. The current study indicates that even at much lesser duration (~2 min 10 sec), flow starts decreasing in the vessel and perforators distal to occlusion. Multiple application of temporary clip further hampers the flow distal to it. The current study suggests applying shorter duration clips with longer intervals between two clip applications. The number of temporary clips applied should also be cut short to as minimum as possible.
There are certain limitations of the current study. ICG-VA is restricted only to vessels visible in the microscope, but not all perforating arteries may be visible during the moment of ICG-VA. There were cases in which the clip(s) hampered the view and cases with perforating arteries originating from the contralateral wall of the vessel that might have been missed. The manipulation of the vessels during the microdissection procedure and the use of retractors in some cases with subarachnoid hemorrhage, swelling, and intraoperative aneurysm rupture may have led to a temporary compression and occlusion of a perforating artery beyond the ischemic tolerance time. When the retractor is removed or repositioned before ICG-VA, the occluded perforating artery reopens and may thus appear patent during ICG-VA. Another limitation, although unlikely, severe stenosis leading to insufficient flow for the brain perfusion cannot be totally excluded out.
In this study, although Hunt and Hess grade was related to clinical outcome, no significant association could be established between ischemia and Hunt and Hess grade. A previous study, however found significant association of higher Hunt and Hess grade with ischemia [12]. This discrepancy may have resulted from smaller number of cases in our study design.
Patients undergoing clipping for large aneurysms are generally considered to have a poor outcome than patients with small aneurysms as patients with ruptured large aneurysms tend to have a poor clinical condition on admission. However, the current study could not establish any such association. This could again be due to lesser number of patients recruited.
In aneurysm surgery, we often need a combination of different tools and methods to ensure that the aneurysm is occluded and that no vessel is compromised. Therefore, ICG-VA should be considered as one tool among others, such as visual inspection, intraoperative digital subtraction angiography, microvascular doppler and electrophysiological monitoring.
The current study assessed the effect of TAO during aneurysm surgery on hemodynamic parameters and cerebral flow as no previous studies recorded these parameters although use of ICG-VA has now become a norm. The only hemodynamic parameter which showed significant change during TAO is increasing blood pressure, which actually may be protecting the brain from ischemic effects of TAO. The significant variables associated with postoperative ischemic changes are duration and number of times temporary clips used. ICG-VA during aneurysm surgery is a simple yet highly powerful instrument that can warn the surgeon of impending ischemia during TAO. Many a times the importance of assessing the vascular flow after removal of the temporary clips is overlooked and this study urges the neurosurgeons to assess the vascular territory of interest both before and after the application of temporary clips so that chances of ischemia can be minimized.
REFERENCES
1. Chandler JP, Getch CC, Batjer HH. Intraoperative aneurysm rupture complication avoidance. Neurosurg Clin N Am. 1998; Oct. 9(4):861–8.
2. Charbel FT, Ausman JI, Diaz FG, Malik GM, Dujovny M, Sanders J. Temporary clipping in aneurysm surgery: technique and results. Surg Neurol. 1991; Aug. 36(2):83–90.

3. David CA, Prado R, Dietrich WD. Cerebral protection by intermittent reperfusion during temporary focal ischemia in the rat. J Neurosurg. 1996; Nov. 85(5):923–8.

4. de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2008; Jun. 62(6 Suppl 3):1300–10.

5. Doss VT, Goyal N, Humphries W, Hoit D, Arthur A, Elijovich L. Comparison of intraoperative indocyanine green angiography and digital subtraction angiography for clipping of intracranial aneurysms. Interv Neurol. 2015; Jul. 3(3-4):129–34.

6. Fortuny LA, Adams CB, Briggs M. Surgical mortality in an aneurysm population: effects of age, blood pressure and preoperative neurological state. J Neurol Neurosurg Psychiatry. 1980; Oct. 43(10):879–82.

7. Ha SK, Lim DJ, Seok BG, Kim SH, Park JY, Chung YG. Risk of stroke with temporary arterial occlusion in patients undergoing craniotomy for cerebral aneurysm. J Korean Neurosurg Soc. 2009; Jul. 46(1):31–7.

8. Jabre A, Symon L. Temporary vascular occlusion during aneurysm surgery. Surg Neurol. 1987; Jan. 27(1):47–63.

9. Kassell NF, Drake CG, Peerless SJ. Occlusion of major cerebral arteries: new concepts. Surg Forum. 1978; 29:527–9.
10. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP. The international cooperative study on the timing of aneurysm surgery. Part 2: Surgical results. J Neurosurg. 1990; Jul. 73(1):37–47.
11. Lanzino G, Kassell NF, Germanson TP, Kongable GL, Truskowski LL, Torner JC, et al. Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse? J Neurosurg. 1996; Sep. 85(3):410–8.

12. Momma F, Wang AD, Symon L. Effects of temporary arterial occlusion on somatosensory evoked responses in aneurysm surgery. Surg Neurol. 1987; Apr. 27(4):343–52.

13. Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell RM. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996; May. 84(5):785–91.

14. Ojemann RG, Heros RC, Crowell RM. Intracranial aneurysms: General aspects of surgical treatment. In : Ginsberg M, Bogasslavhsy B, editors. Surgical Management of Cerebrovascular Disease. Williams & Wilkins Baltimore,;1988. p. 163–77.
15. Aneurysms of the anterior communicating artery. Bifrontal craniotomy and routine use of temporary clips. J Neurosurg. 1961; Jan. 18:98–112.
16. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003; Jan. 52(1):132–9. discussion 139.

17. Selvapandian S, Sudarsan PS, Shaji PG, Chandy MJ. Elective intermittent temporary clipping in aneurysm surgery: A practical protocol. Indian J Neurosurg. 2015; 4(1):8–14.
18. Sonesson B, Ljunggren B, Säveland H, Brandt L. Cognition and adjustment after late and early operation for ruptured aneurysm. Neurosurgery. 1987; Sep. 21(3):279–87.

19. Sugita K. Microneurosurgical atlas. Berlin: Springer-Verlag;1985. p. 10–14.
20. Symon L. European Association of Neurosurgical Societies Fifth European lecture. Barcelona, February 24, 1984. Thresholds of ischaemia applied to aneurysm surgery. Acta Neurochir. 1985; 77(1-2):1–7.
Fig. 1.
The time gap of 1 second between appearance of ICG and complete opacification of vessels when no clip was applied was increased to 3 seconds after application of 1st temporary clip that was in place for 3 min 23 seconds. ICG, indocyanine green.
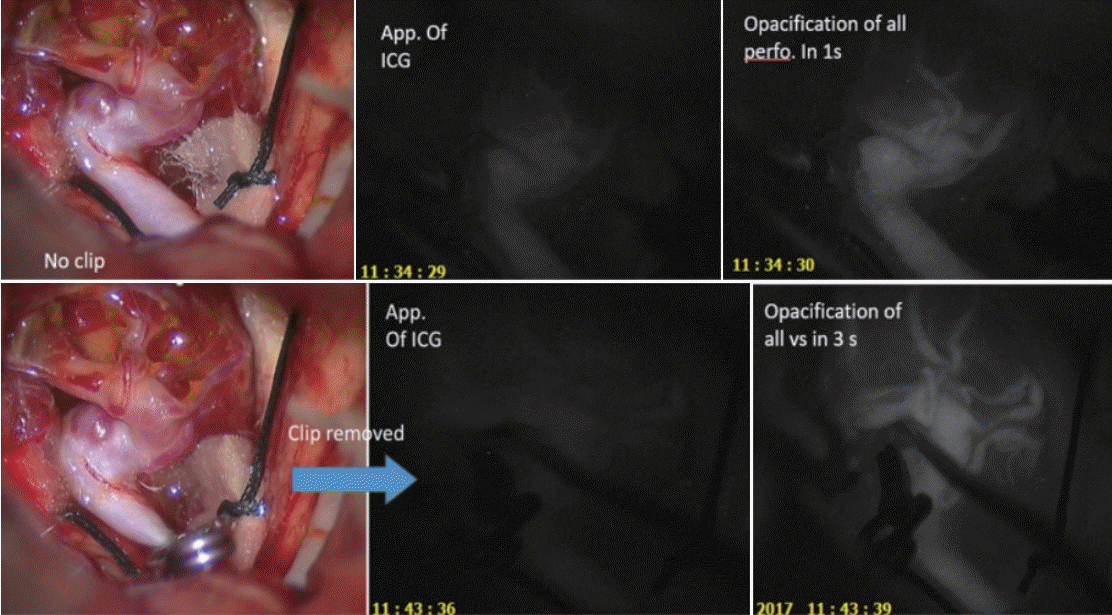
Fig. 2.
Acom artery aneurysm. (A) shows one perforator arising in the vicinity of the aneurysm visible on ICG-VA image before applying any temporary clip. (B) shows the ICG-VA image taken after removal of the 4th temporary clip that was applied for 5 min 30 sec. The perforator is not opacified anymore. ICG-VA, indocyanine green video angiography
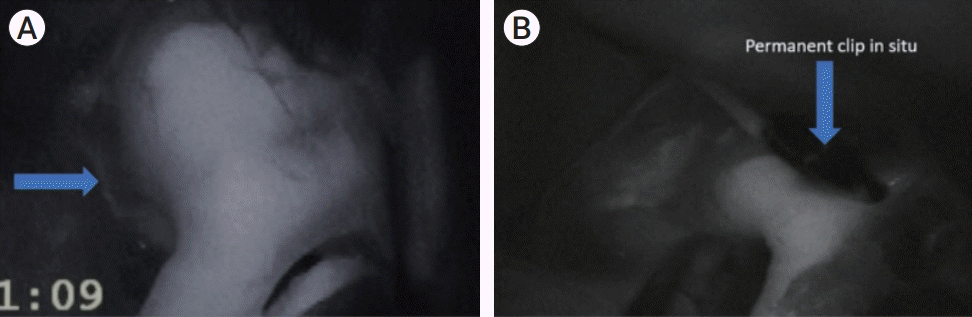
Fig. 3.
Right A1-A2 junction aneurysm. (A) shows the ICG-VA image before applying any temporary clip where 3 perforators arising in the vicinity of Rt ACA can be seen. (B) shows the ICG-VA image taken after removal of 3rd temporary clip that was applied for 7 min 2 second revealing only one remaining perforator. ICG-VA, indocyanine green video angiography; ACA, anterior cerebral artery.
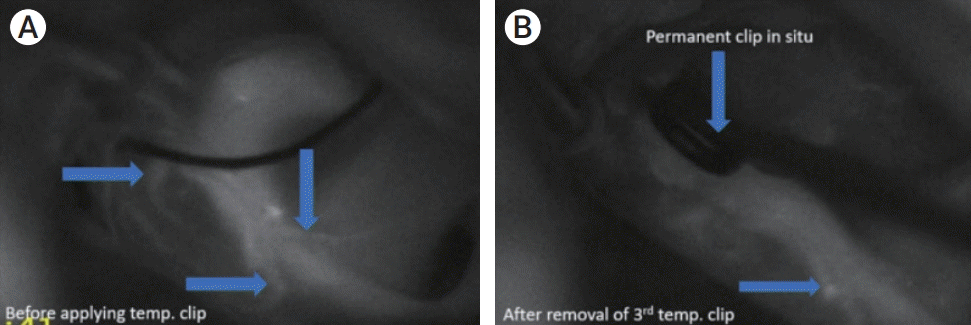
Table 1.
Baseline characteristics of the patients and duration of temporary clips applied
Table 2.
Time interval between application of temporary clips (in seconds)
Table 3.
Average delay in appearance of ICG after application of temporary clip (in seconds) Bold type represents significant values (p<0.05)
Table 4.
Physiological variations during temporary clipping
Table 5.
Characteristics of 9 patients showing symptomatic (S. No. 1-6) and asymptomatic (S. No. 7-9) ischemic changes
WFNS, World Federation of Neurosurgical Societies; ICG, indocyanine green; DW-MRI, diffusion weighted magnetic resonance image; Acomm, anterior communicating; MCA, middle cerebral artery; ACA, anterior cerebral artery; DACA, distal anterior cerebral artery; ICA-PComm. internal carotid artery-posterior communicating




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download