Abstract
We report two rare cases treated with coiling after rapid regrowth (within a month) of an aneurysm remnant on the middle cerebral artery (MCA) trunk after incomplete surgical clipping. The first case, a 47-year-old man with subarachonoid hemorrhage (SAH) (Hunt-Hess grade II, Fisher grade III) underwent clipping of a ruptured saccular aneurysm with a wide neck on the right early frontal branch arising from the MCA trunk. Incomplete clipping with a 1 mm sized remnant neck was performed to avoid sacrificing the lenticulostriate artery. In a follow-up cerebral angiogram on postoperative day 30, a rapid regrowth of the aneurysm remnant was observed, and on that day, complete obliteration was obtained by rescue endovascular treatment. The second case, a 48-year-old healthy woman with SAH (Hunt-Hess grade II, Fisher grade III) underwent clipping of an anteroposteriorly projecting bilobulated aneurysm on the left M1. Incomplete clipping with a minimal remnant neck was performed. In follow-up digital subtraction angiogram on postoperative day 30, a rapid regrowth of an aneurysm remnant involving only a part of the initial aneurysm near the neck was observed, and on that day, complete obliteration was obtained by rescue coiling. These patients were both discharged without any neurological deficits.
Aneurysm remnant (AR) after surgical clipping of ruptured aneurysms could be at persistent risk of rebleeding [3-6]. Secondary coiling after incomplete clipping represents a strategy to occlude the AR. According to previous reports, in cases of a complete clipped intracranial aneurysm, the annual risk of recurrence is very low (0.02-0.52%) [2,5-7]. In contrast, in cases of an incomplete clipped intracranial aneurysm with AR, it is substantially higher (0.38 to 7.3%) [4,7,8]. Thus, neurosurgeons have come to a consensus that long-term follow up in patients with AR after incomplete clipping is needed. Generally, blister type aneurysms or dissecting aneurysms have a thin aneurysmal wall, broad-based on the parent artery, and are thus prone to easy premature rupture during the operation and can result in a rapid regrowth of the AR after treatment [1,3,9]. However, risk factors for the regrowth of AR after incomplete obliteration have not been established and also no protocol has been established that defines the timing or optimal modality of follow-up imaging [7].
To the best of my knowledge, cases of the rapid regrowth (within a month) of AR on the on middle cerebral artery (MCA) trunk after unsuccessful surgical clipping in patients with a ruptured cerebral aneurysm have rarely been reported. Here, we report two rare cases of rescue endovascular treatment for AR on the MCA trunk after incomplete surgical clipping.
A 47-year-old man presented after the sudden onset of a headache without neurological deficits (Hunt–Hess grade II). Brain computed tomography (CT) scan demonstrated a diffuse subarachnoid hemorrhage (Fisher grade III). 3D reconstruction images of brain computed tomography angiogram (CTA) revealed a 3.9 mm saccular aneurysm with a wide neck and its neck was incorporated into the early frontal branch on the early frontal branch of the right MCA (Fig. 1A). Instead of an endovascular treatment, we performed craniotomy and neck clipping because the wide neck of aneurysm was incorporated with both divisions. The aneurysm was deep and proximal in the sylvian cistern. During dissection and clipping, the adhesion between the posterior aneurysm wall and the lenticulostriate artery (LSA) were a special concern. Incomplete clipping with a minimal remnant neck was unavoidably performed to avoid sacrificing the LSA. We could not check the results by intraoperative indocyanine green videoangiography (ICG-VA) because the location of the AR was behind the clipped aneurysm. Immediate postoperative CTA demonstrated a 1 mm AR behind the clipped aneurysm (Fig. 1B, C). During the postoperative period, he had no hydrocephalus or medically refractory delayed cerebral vasospasm.
In follow-up digital subtraction angiography (DSA) at postoperative day 30, a rapid regrowth of an AR (two blebs, laterally projecting, depth: 7.39 mm×width: 2.58 mm×neck: 3.01 mm) was seen (Fig. 1D). On that day, we performed coil embolization of the AR and complete obliteration was obtained (Fig. 1E). He was discharged without any neurological deficits at postoperative day 34. At 6- and 18-month follow-up, DSA studies demonstrated minimal recanalization due to compaction of the coil.
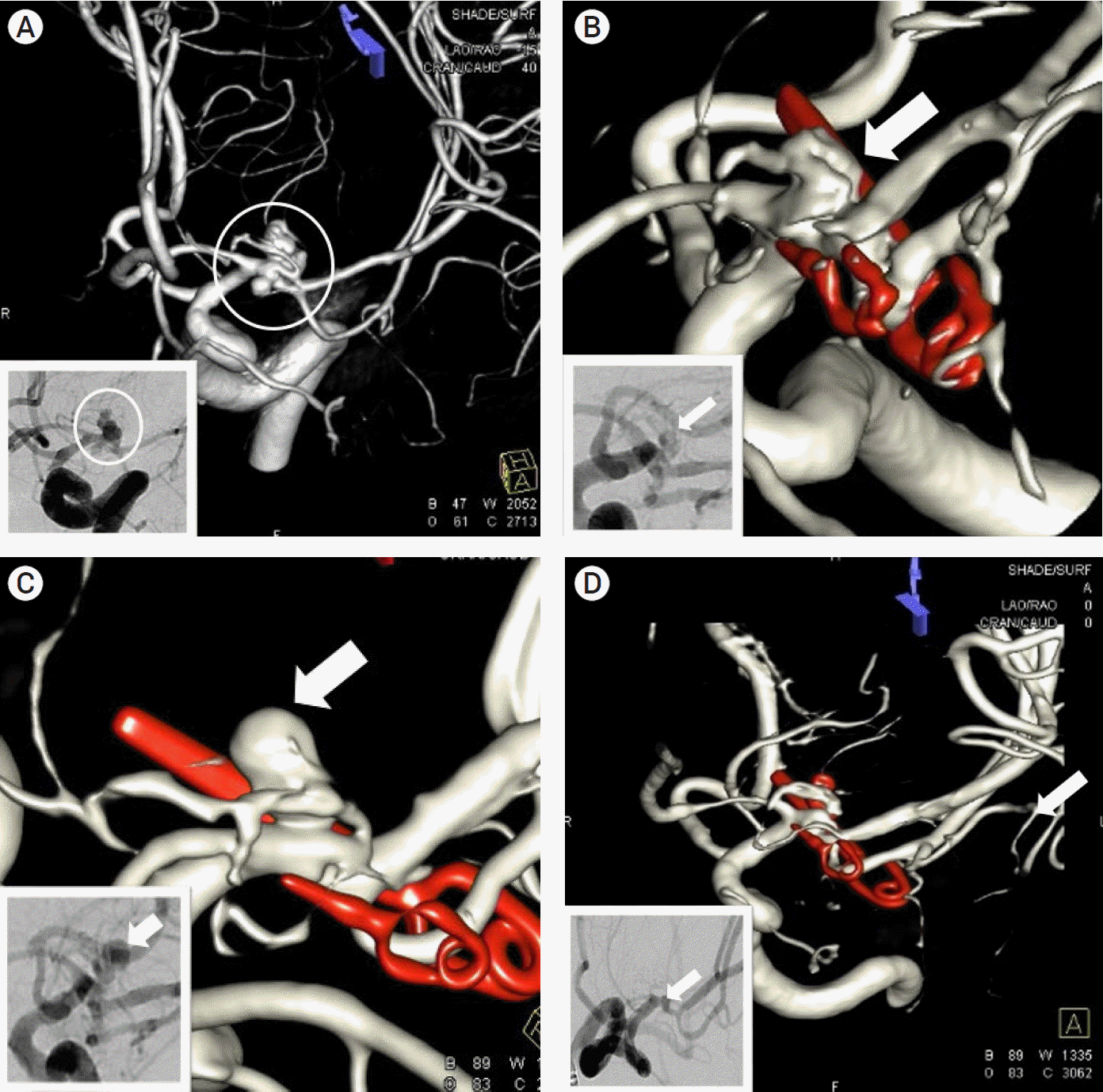
A 48-year-old woman presented after the sudden onset of a headache without neurological deficits (Hunt–Hess grade II). Brain CT demonstrated a diffuse subarachnoid hemorrhage (Fisher grade III). A preoperative DSA showed a ruptured kissing aneurysm (maximal diameter 8 mm) with an anteroposteriorly projecting bilobulated figure and its neck was incorporated with the LSA on the left MCA trunk (Fig. 2A). We performed a craniotomy and neck clipping because the complex figure of the aneurysm could not provide a working view for endovascular treatment. The aneurysm was deep and proximal in the sylvian cistern. During dissection and clipping, the adhesion between a posterior portion of the aneurysm and the LSA was a special concern. Incomplete clipping with minimal AR was performed to avoid sacrificing the LSA. Immediate postoperative DSA demonstrated that a 0.6 mm×3.2 mm AR was located between the LSA and the clipped posterior lobar aneurysm (Fig. 2B).
We thought it best to observe the AR because the size and figure of the AR had a low risk of rebleeding. During the postoperative period, she developed medically refractory delayed cerebral vasospasm. On postoperative days 7, 8, and 9 after clipping, endovascular treatment with intra-arterial nimodipine infusion was performed. During endovascular treatment for the spasm, DSA showed an unchanged AR.
In follow-up DSA at postoperative day 30, a rapid regrowth of the AR (one bleb, superiorly projecting, 7.10 mm ×width: 3.48 mm×neck: 3.21 mm) was seen (Fig. 2C). On that day, we performed coil embolization of the AR and complete obliteration was obtained (Fig. 2D). She was discharged without any neurological deficits at postoperative day 36. At 3 months follow-up, DSA studies demonstrated the complete obliteration of the aneurysm.
In past studies, the average duration to regrowth of an AR after clipping was about ten years [1,2,4,12]. Also, according to a recent study, the rate of regrowth was likely slow, less than 0.5 mm per year, which justified following up with DSA 3 to 5 years after microsurgical clipping [13]. Although there is general late recurrence and a slow growth rate of AR after clipping, our two cases described ARs on the MCA trunk that rapidly re-grew within a month as a rapid changing radiologic presentation of dissecting aneurysm. Although no clear mechanism has been known for the rapid regrowth of AR, it was generally thought to be due to arterial wall weakening and/or the influence of hemodynamics stress [11-14]. Similarly, our hypothesis is that a change of focal hemodynamic stress by microsurgical clipping may be one of the main factors that leads to decreasing resistibility and structural fragility of the AR wall. The change of hemodynamic stress not only influences the rapid regrowth of an AR arising from the MCA trunk but also the anatomical location of the proximal MCA.
Generally, compared with the MCA, higher rates of unsuccessful clipping are associated with aneurysms located at the anterior communicating artery complex or posterior circulation [6-9,12]. However, MCA trunk aneurysms are located either on the main trunk, at the origin of an early frontal or temporal branch, or at the origin of the lateral LSA. Although the incidence of aneurysms of the MCA trunk (from its origin up to the bifurcation) is very low (2% to 7% of total aneurysms) [1,7,12-14] this location of an aneurysm usually makes the operation more difficult, since in a high percentage of cases they are broad-necked, and in general, the origin of these branches is firmly incorporated within the neck, and sometimes its visualization is hidden by the aneurysm itself. These features of proximal MCA aneurysms could make microsurgical clipping difficult or unsuccessful.
When the regular shape and small size of the aneurysm remnants after initial clipping was identified via post-clipping imaging (CTA or 3D-DSA), we could not know exactly the need for retreatment (prompt or delayed). Thus we judged that it had a low risk of rebleeding or regrowth. So we determined a short term follow-up and observation. The rapid regrowth of AR is assumed to be due to the decreased resistibility and structural fragility of the AR wall, and it can result in serious and fatal consequences like the natural history of a ruptured blood blister aneurysm [4,5,9]. Against expectation, prompt treatment should be considered when rapid regrowth of AR with putative high re-rupture risk are identified. Before the development of endovascular treatment, re-operation for an AR was the only option for treatment. However, additional clipping or replacement of a clip are usually more difficult due to changes in the anatomical structure or adhesions in the operated area (in particularly for proximal MCA) and this increases morbidity and mortality [1-4]. Lately, endovascular coil embolization has become an alternative treatment modality for AR. Many previous studies have reported that endovascular treatment of AR after surgical clipping has favorable outcomes, suggesting that endovascular coil embolization may be a safe and reliable treatment option [2,13]. In our two cases, fortunately, the neck of the AR was narrow because of neck remodeling as a result of the clipping, and prompt coil embolization was performed as a rescue treatment when the rapid regrowth of the remnant aneurysm was identified.
In summary, many reports have indicated that size, morphology, clip reconstruction technique, flow dynamics, female sex and younger age (less than 45 years) predisposes a patient to regrowth of an AR. Nevertheless, it remains controversial and partially unknown whether aneurysm-specific factors and patient-specific factors can influence the regrowth of AR. When intraoperative assessment is shown to be insufficient to predict clipped AR, we usually perform a routine post-clipping imaging (CTA or 3D-DSA) after aneurysm clipping. The main purpose of post-clipping image was to eliminate the risk of a rebleed from an AR by allowing follow-up and/or retreatment. Like our two patients with subarachonoid hemorrhage, the early detection of an AR was possible because of postoperative image work-up. Unfortunately, magnetic resonance angiography or 3-dimensional CT of the cerebral arteries is less likely to be feasible in patients with a clip artifact. Whether the risk of DSA in follow-up studies is acceptable may be controversial, but a recent report in which meta-analysis was used estimated a low risk of DSA, which seems to justify DSA as an acceptable examination in the detection of re-growing AR [1,6,12]. We thought that higher detection rates of small ARs are now more likely because of postoperative 3D-DSA and prompt endovascular treatment is possible following DSA. Also, special attention to younger patients, anatomical location of aneurysms, unsuccessful clipping and unintended remnants of any size is essential. In such a constellation of risk factors, early postoperative imaging follow-up within a month after clipping may be warranted to rule out a small but rapid regrowth of the AR.
In two rare cases, the location (MCA trunk) of the aneurysm remnant after microsurgical clipping might have been associated with changes of hemodynamic stress and this might have led to their rapid regrowth because of the structural fragility of the aneurysmal wall. To determine when to perform a follow-up angiogram to detect rapid regrowth of a small aneurysm remnant like in our cases is vital.
REFERENCES
1. Burkhardt JK, Chua MHJ, Weiss M, Do AS-MS, Winkler EA, Lawton MT. Risk of aneurysm residual regrowth, recurrence, and de novo aneurysm formation after microsurgical clip occlusion based on follow-up with catheter angiography. World Neurosurg. 2017; Oct. 106:74–84.

2. Cekirge HS, Islak C, Firat MM, Kocer N, Saatci I. Endovascular coil embolization of residual or recurrent aneurysms after surgical clipping. Acta Radiol. 2000; Mar. 41(2):111–5.

3. Dashti R, Rinne J, Hernesniemi J, Niemelä M, Kivipelto L, Lehecka M, et al. Microneurosurgical management of proximal middle cerebral artery aneurysms. Surg Neurol. 2007; Jan. 67(1):6–14.

4. Della Pepa GM, Bianchi F, Scerrati A, Albanese A, Cotroneo E, Delitala A, et al. Secondary coiling after incomplete surgical clipping of cerebral aneurysms: a rescue strategy or a treatment option for complex cases? Institutional series and systematic review. Neurosurg Rev. 2019; Jun. 42(2):337–50.

5. El Beltagy M, Muroi C, Roth P, Fandino J, Imhof H-G, Yonekawa Y. Recurrent intracranial aneurysms after successful neck clipping. World Neurosurg. 2010; Oct-Nov. 74(4-5):472–7.

6. Jun HS, Ahn J, Song JH, Chang IB. Spontaneous regression of aneurysm remnant after incomplete surgical clipping in a patient with ruptured cerebral aneurysm. J Cerebrovasc Endovasc Neurosurg. 2016; Dec. 18(4):402–6.

7. Kivisaari RP, Porras M, Ohman J, Siironen J, Ishii K, Hernesniemi J. Routine cerebral angiography after surgery for saccular aneurysms: is it worth it? Neurosurgery. 2004; Nov. 55(5):1015–24.

8. Kobayashi S, Moroi J, Hikichi K, Yoshioka S, Saito H, Tanabe J, et al. Treatment of Recurrent Intracranial Aneurysms After Neck Clipping: Novel Classification Scheme and Management Strategies. Oper Neurosurg (Hagerstown). 2017; Dec. 13(6):670–8.

9. Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg. 1989; Apr. 70(4):556–60.

10. Park JC, Shim JH, Lee DH, Ahn JS, Lee DG, Yang K, et al. Three-dimensional angiographic evaluation of middle cerebral artery trunk aneurysms: demonstration of the close relationship between the early frontal cortical branches and lateral lenticulostriate arteries. World Neurosurg. 2016; Jul. 91:383–9.

11. Ravindra VM, Karsy M, Schmidt RH, Taussky P, Park MS, Bollo RJ. Rapid de novo aneurysm formation after clipping of a ruptured middle cerebral artery aneurysm in an infant with an MYH11 mutation. J Neurosurg Pediatr. 2016; Oct. 18(4):463–70.

12. Spiotta AM, Hui F, Schuette A, Moskowitz SI. Patterns of aneurysm recurrence after microsurgical clip obliteration. Neurosurgery. 2013; Jan. 72(1):65–9. discussion 69.

13. Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. 2001; May. 32(5):1191–4.
Fig. 1.
A 47-year-old man patient (Hunt- Hess grade II) with subarachnoid hemorrhage (Fisher grade III). Initial three-dimensional reconstruction images in brain computed tomographic angiogram (CTA) revealed a 3.9 mm sized saccular aneurysm (white arrow) with wide neck and it`s neck was incorporated with early frontal branch on right early frontal cortical branch of middle cerebral artery trunk (A). Immediate postoperative CTA demonstrated about 1 mm sized aneurysm remnant (AR, white arrow) behind a clipped aneurysm (white circle) (B: posterior view, C: magnified image in small box). In follow-up digital subtraction angiography (DSA) at postoperative day 30, a rapid regrowth of AR (white arrow) with two blebs (laterally projecting and depth: 7.39 mm×width: 2.58 mm×neck: 3.01 mm) was showed (D: magnified 180˚ reverse image in small box). A prompt endovascular treatment was done and complete obliteration (white arrow) of AR was done (E: magnified DSA working image in small box).
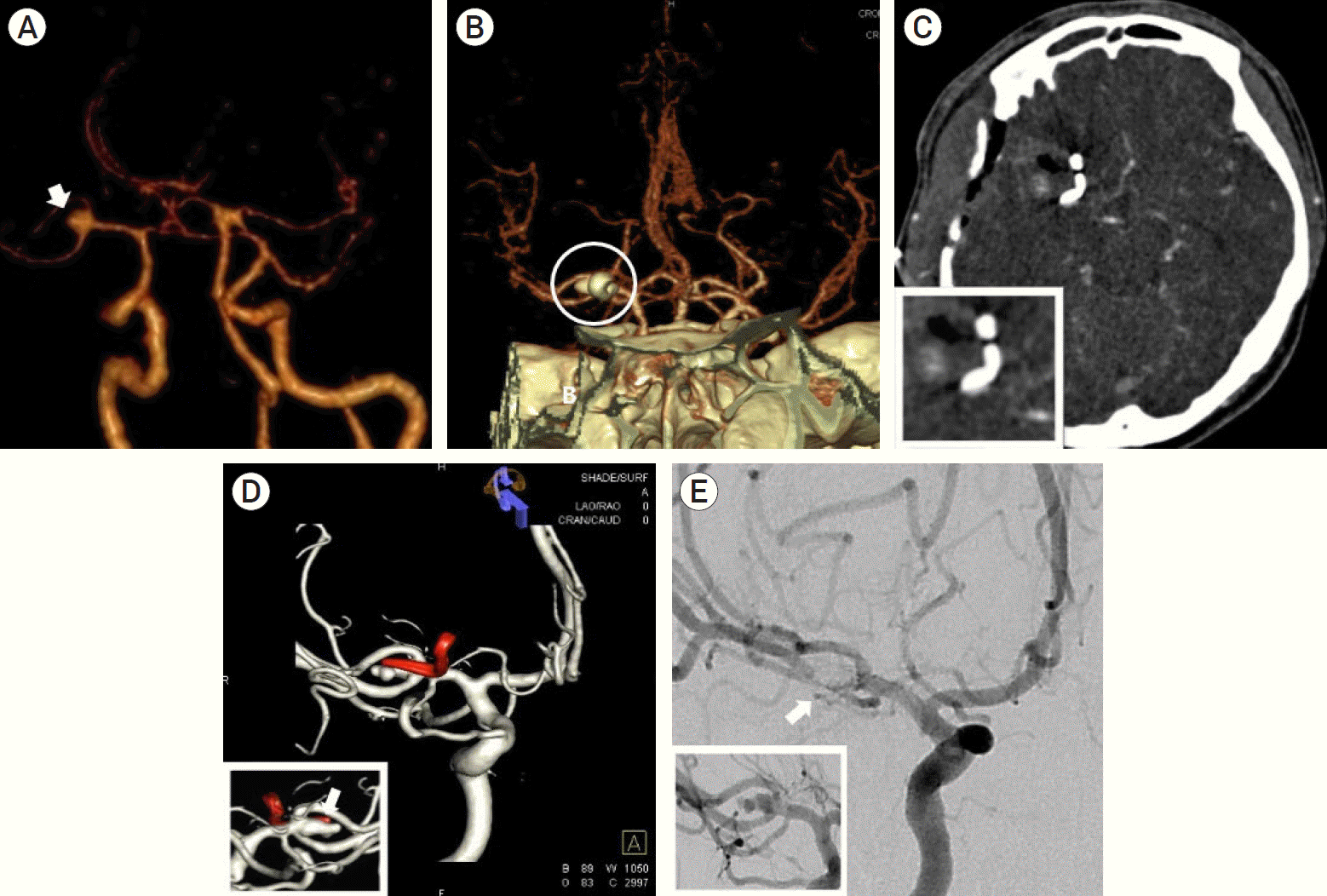
Fig. 2.
A 48-year-old woman patient (Hunt- Hess grade II) with subarachnoid hemorrhage (Fisher grade III). Three-dimensional reconstruction image in initial digital subtraction angiography (DSA) showed a ruptured 8 mm sized aneurysm (white circle) with anteroposteriorly projecting bilobar figure and it`s neck was incorporated with lateral lenticulostriate artery (LSA) on left middle cerebral artery trunk (A: magnified DSA image in small box). Immediate postoperative three-dimensional -DSA demonstrated 0.6 mm x 3.2 mm sized remnant aneurysm (AR, white arrow) was located between LSA and a clipped posterior portion aneurysm (AR, white arrow) (B: magnified DSA image in small box). In follow-up three-dimensional -DSA at postoperative day 30, a rapid regrowth of AR (white arrow) with one bleb, superiorly projecting and depth: 7.10 mm×width: 3.48 mm×neck: 3.21 mm was showed (C: magnified DSA image in small box). A prompt endovascular treatment was done and complete obliteration (white arrow) of AR was done (D: magnified DSA working image in small box). At 3 months follow-up, DSA studies demonstrated complete obliteration of aneurysm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download