1. Reichel LM, Morales OA. Gross anatomy of the elbow capsule: a cadaveric study. J Hand Surg Am. 2013; 38:110–6.

2. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006; 34:1926–32.

3. Paletta GA Jr, Wright RW. The modified docking procedure for elbow ulnar collateral ligament reconstruction: 2-year follow-up in elite throwers. Am J Sports Med. 2006; 34:1594–8.
4. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002; 30:541–8.

5. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001; 10:152–7.

6. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991; 175:203–12.
7. Williams PL, Warwick R. Gray’s anatomy. 36th ed. Edinburgh: Churchill Livingsone;1980.
8. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983; 11:315–9.

9. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991; 73:440–6.
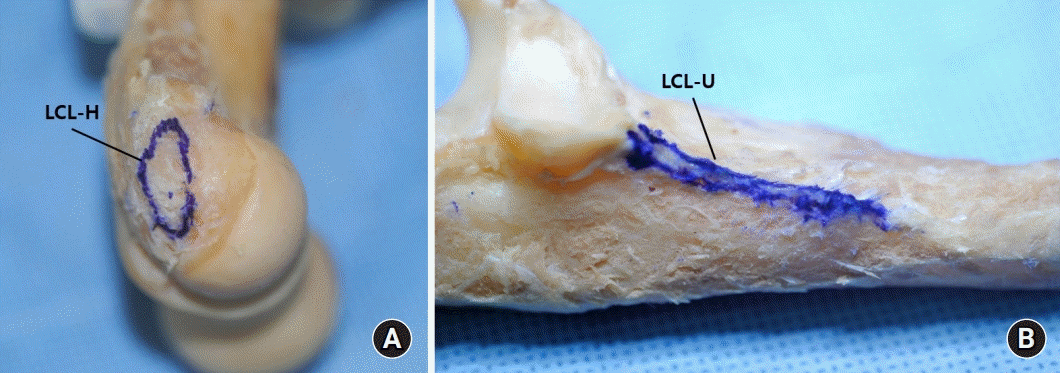
10. Olsen BS, Vaesel MT, Søjbjerg JO, Helmig P, Sneppen O. Lateral collateral ligament of the elbow joint: anatomy and kinematics. J Shoulder Elbow Surg. 1996; 5(2 Pt 1):103–12.

11. Jarrett CD, Weir DM, Stuffmann ES, Jain S, Miller MC, Schmidt CC. Anatomic and biomechanical analysis of the short and long head components of the distal biceps tendon. J Shoulder Elbow Surg. 2012; 21:942–8.

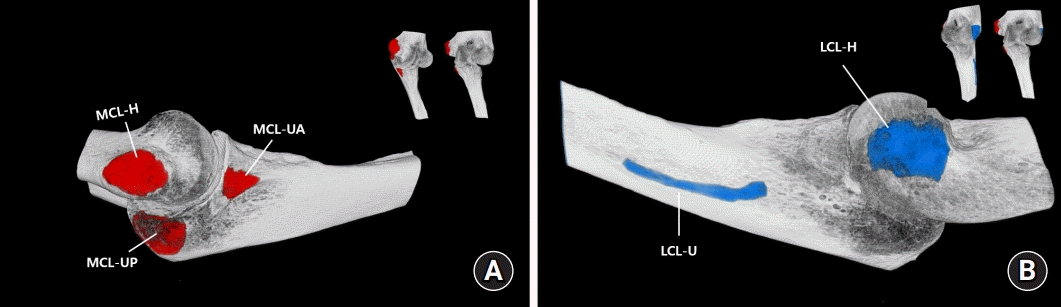
12. Capo JT, Collins C, Beutel BG, et al. Three-dimensional analysis of elbow soft tissue footprints and anatomy. J Shoulder Elbow Surg. 2014; 23:1618–23.

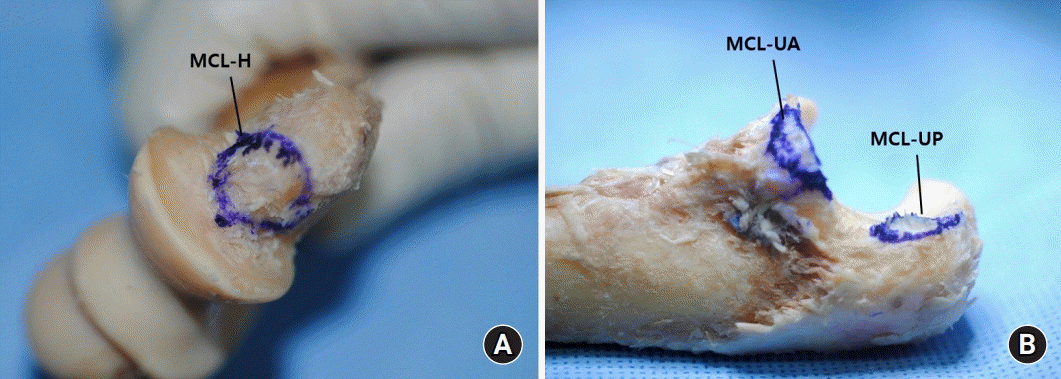
13. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007; 16:657–60.

14. Miyake J, Oka K, Moritomo H, Sugamoto K, Yoshikawa H, Murase T. Open reduction and 3-dimensional ulnar osteotomy for chronic radial head dislocation using a computer-generated template: case report. J Hand Surg Am. 2012; 37:517–22.

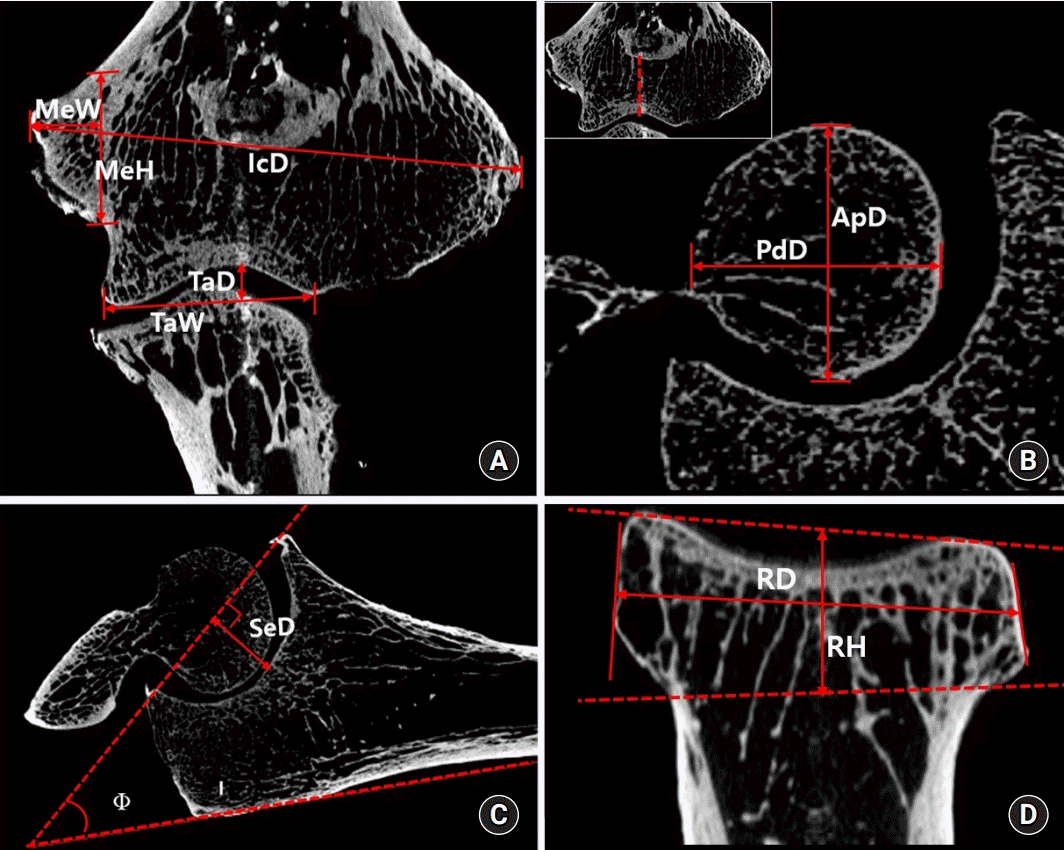
15. Hsu JT, Chen YJ, Ho JT, et al. A comparison of micro-CT and dental CT in assessing cortical bone morphology and trabecular bone microarchitecture. PLoS One. 2014; 9:e107545.

16. Hsu JT, Wang SP, Huang HL, Chen YJ, Wu J, Tsai MT. The assessment of trabecular bone parameters and cortical bone strength: a comparison of micro-CT and dental cone-beam CT. J Biomech. 2013; 46:2611–8.

17. Jenkins PJ, Ramaesh R, Pankaj P, et al. A micro-architectural evaluation of osteoporotic human femoral heads to guide implant placement in proximal femoral fractures. Acta Orthop. 2013; 84:453–9.

18. Norman DG, Getgood A, Thornby J, et al. Quantitative topographic anatomy of the femoral ACL footprint: a micro-CT analysis. Med Biol Eng Comput. 2014; 52:985–95.

19. Rozental TD, Deschamps LN, Taylor A, et al. Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am. 2013; 95:633–42.

20. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006; 34:362–9.

21. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979; 11:447–55.

22. Wan C, Hao Z, Wen S. A quantitative comparison of morphological and histological characteristics of collagen in the rabbit medial collateral ligament. Ann Anat. 2013; 195:562–9.

23. Barco R, Antuña SA. Management of elbow trauma: anatomy and exposures. Hand Clin. 2015; 31:509–19.
24. Farrow LD, Mahoney AJ, Stefancin JJ, Taljanovic MS, Sheppard JE, Schickendantz MS. Quantitative analysis of the medial ulnar collateral ligament ulnar footprint and its relationship to the ulnar sublime tubercle. Am J Sports Med. 2011; 39:1936–41.

25. O’Driscoll SW, Morrey BF, Korinek SL, An KN. Elbow joint instability: A kinematic model. J Shoulder Elbow Surg. 1994; 3:143–50.
26. O’Driscoll SW, Morrey BF, Korinek S, An KN. Elbow subluxation and dislocation. A spectrum of instability. Clin Orthop Relat Res. 1992; (280):186–97.
27. Hart RT, Hennebel VV, Thongpreda N, Van Buskirk WC, Anderson RC. Modeling the biomechanics of the mandible: a three-dimensional finite element study. J Biomech. 1992; 25:261–86.

28. Liu SH, Osti L, Henry M, Bocchi L. The diagnosis of acute complete tears of the anterior cruciate ligament. Comparison of MRI, arthrometry and clinical examination. J Bone Joint Surg Br. 1995; 77:586–8.
29. Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978; 203:305–21.
30. Cage DJ, Abrams RA, Callahan JJ, Botte MJ. Soft tissue attachments of the ulnar coronoid process. An anatomic study with radiographic correlation. Clin Orthop Relat Res. 1995; (320):154–8.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download