INTRODUCTION
The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is characterized by a broad clinical spectrum ranging from asymptomatic to severe viral pneumonia with respiratory failure and even death.
1) COVID-19 has become a global crisis as it spread worldwide, taking millions of lives and causing disastrous socio-economic impact. A COVID-19 infection causes right and left ventricular dilation, myocardial fibrosis, and myocarditis, necessitating immediate treatment.
2) However, treatments for COVID-19 infection can produce adverse cardiovascular effects, including a prolonged QT interval and the development of arrhythmias.
3)
COVID-19 mortality is often related to respiratory failure caused by severe acute pneumonia, systemic inflammatory response syndromes like cytokine storms, and myocardial injuries such as acute fulminant myocarditis with arrhythmias.
4) In this regard, cardiac injuries associated with COVID-19 infection are emerging as a significant issue. Some authors reported that COVID-19 infection caused repolarization abnormalities and prolonged QT intervals.
2)5)6)7) Furthermore, prolonged QT interval was associated with higher mortality.
6)
To address these issues, this study aims to determine the characteristic electrocardiogram (ECG) findings according to clinical severity in patients with COVID-19 infection. Moreover, this study evaluated the ECG parameters associated with mortality.
METHODS
Ethical statement
The study was conducted according to the ethical guidelines of the Declaration of Helsinki revised in 2013. This study was approved by the Daegu Joint Institutional Review Board (DGIRB 2020-07-007-002). Written informed consent was waived because of the retrospective nature of this study.
Study design
Between February and April of 2020, 7 medical centers in Daegu, South Korea, admitted 822 patients confirmed to have COVID-19 via real-time reverse transcriptase-polymerase chain reaction assay testing of nasal or pharyngeal swabs. Among them, 267 patients who underwent an ECG test after admission were examined, and patients who were below 18 years old, used psychotropic agents, and exhibited a history of mental disorders were excluded (
Supplementary Figure 1).
The study collected medical records based on a review of patients' charts retrospectively. Three cardiologists reviewed the clinical severity score of COVID infection through an electronic medical recorder and laboratory data like log N-terminal pro-B type natriuretic peptide (NT-proBNP), C-reactive protein (CRP), cardiac enzyme (creatinine kinase-myocardial band [CK-MB], cardiac troponin I or T), and ECG data such as heart rate, PR interval, QRS duration, QT interval, and Tpe (the interval between peak to end in a T wave).
Clinical severity score
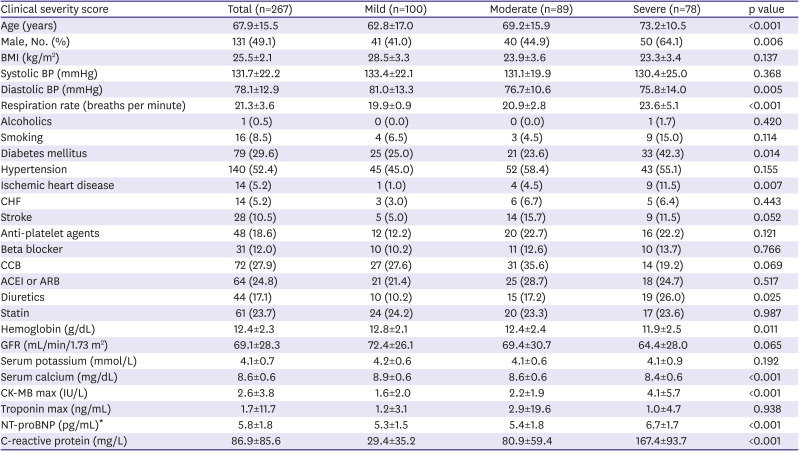
This study used the following scoring system created by the Korean Disease Control and Prevention Agency to determine the severity of a patient's COVID-19 infection. A score of 1 denotes no limit to daily activity; 2 for interfering with daily life, although oxygen treatment was not required; 3 for needing oxygen treatment with nasal prong; 4 for needing oxygen treatment with a facial mask; 5 for needing oxygen treatment with non-invasive ventilation; 6 for needing oxygen treatment with invasive ventilation; 7 for needing oxygen treatment with extracorporeal membrane oxygenation or showing signs of multi-organ failure; and 8 for reported mortality. The patients were divided into three groups using this scoring system, or the clinical severity score: mild group (1–2), moderate group (3–5), and severe group (6–8).
ECG measurement
The ECG was performed in the emergency room or at the time of hospitalization. All ECG parameters (heart rate, PR interval, QRS duration, QT interval, QT dispersion, the interval between peak to end in a T wave [Tpe]) were measured at 10-times magnification using electronic calipers. However, measurements were not performed in cases of excessive artifact or T wave flatness. Measurements were taken at each center and then analyzed and calculated by averaging two different doctors' measurements, respectively, to reduce the inter-observer error. If the ECG rhythm was atrial fibrillation or atrial flutter, each ECG parameter was evaluated as the average of each lead's measured values.
Each QT interval was measured to determine the values of these two extreme indices. For QT measurement, a tangent method was used to indicate the end of the T wave, which is defined as the isoelectric line's intersection with the tangent to the T wave's downslope. Moreover, this study measured the Tpe in all 12 leads. Bazzett's formula was used to generate the corrected QT interval (QTc) and corrected Tpe interval (Tpe-c) because the QT and Tpe interval could change depending on the heart rate.
8) Dispersions of QT and Tpe were calculated as the difference between the longest and shortest QT intervals and Tpe intervals within a 12-lead ECG.
9)
Statistical analysis
Descriptive data were presented as percentages for categorical variables and the mean ± standard deviation for continuous variables of normal distribution. The chi-squared test or Student's t-test performed to compare the two groups was deemed appropriate, and analysis of variance was performed to compare the three groups. Moreover, the area below the receiver operating characteristic (ROC) curve was measured to determine the cut-off point of the highest sensitivity and specificity. Missing value was replaced by missForest package (version 1.04). The univariate analysis' significant variables were entered into the multivariate model, and the associated factors with mortality were identified. For all analyses, the level of significance was set to 0.05, and all reported p values were two-sided. All statistical analyses were performed with R version 3.5.2 software (The R Foundation, Vienna, Austria;
www.R-project.org).
DISCUSSION
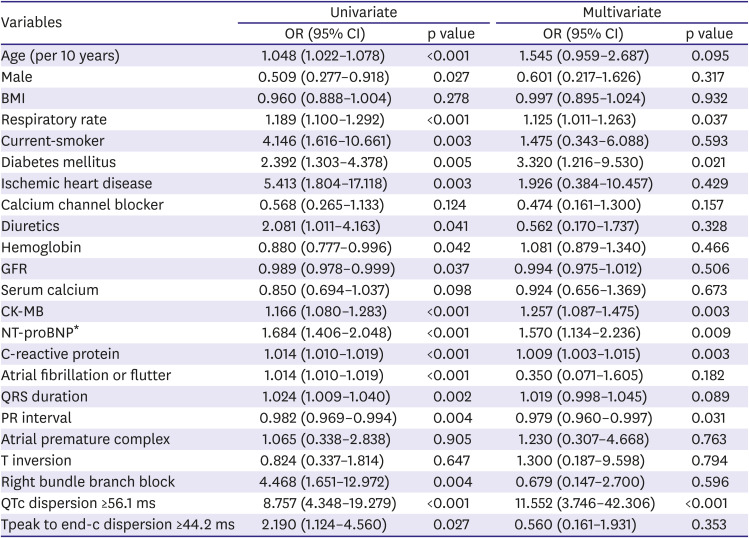
The main findings of the current study are as follows. COVID-19 infection might significantly prolong the QT interval and peak to end of T wave, which stands for repolarization abnormality by cardiac injury. The degree of QT interval prolongation and the peak to the end of the T wave might be related to clinical disease severity in COVID-19 infection. A QTc dispersion of more than 56.1 ms could be an independent factor that can predict mortality.
Several reports have demonstrated that viral infections like adenovirus, coronavirus, and herpes are related to cardiac arrhythmia–like atrial fibrillation due to various inflammatory markers including CRP, tumor necrosis factor-α, and interleukin-2, 6, and 8.
10)11)12) Moreover, some authors reported that COVID-19 could cause cardiac injuries like myocarditis.
5)7)10)12)13) However, it has been acknowledged that data about the mechanism of cardiovascular damage from COVID-19 is limited. Studies have shown that COVID-19 patients with preexisting cardiovascular complications had a higher level of cardiac troponin elevation in their plasma than patients without cardiovascular complications.
2)5) The cardiac troponin level was known to increase in acute myocardial injuries, atherosclerotic plaque disruptions, coronary thromboses, critically ill patients, and supply-demand imbalances leading to myocardial injury, metabolic stress by infection, hypoxia, acidosis, and hypotension.
5)11)14) Moreover, previous studies mentioned that cardiac injury is based on cardiac biomarkers such as cardiac troponin and NT-proBNP.
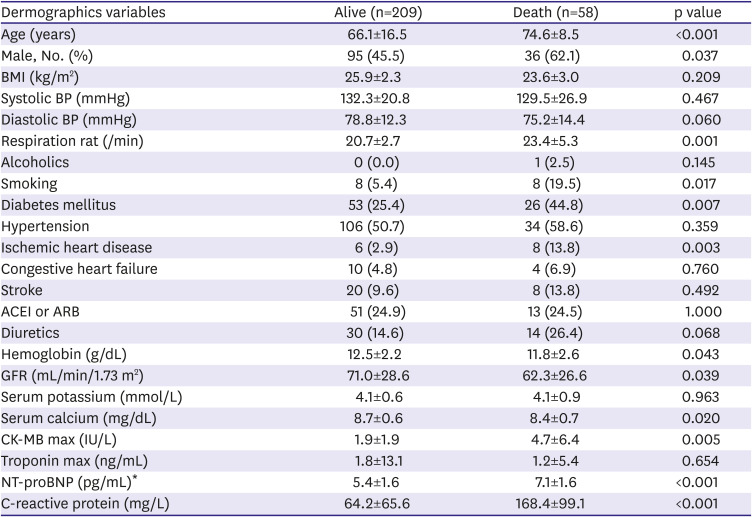
4)5)10) This study found that CK-MB and log NT-proBNP significantly increased in patients with severe COVID-19 conditions than patients with mild conditions, which are similar to previous reports.
Cardiac injuries in COVID-19 infections could be postulated through several mechanisms. The first is the angiotensin-converting enzyme 2 (ACE2)–mediated direct damage.
7)12)15)16)17) ACE2 is an essential regulator of cardiac function and acts as a vasodilator, anti-fibrotic, anti-oxidative, and anti-hypertrophic.
12) ACE2 is also a functional receptor for SARS-CoV, which down-regulates ACE2, contributing to myocardial dysfunction.
12)18) The use of renin-angiotensin-aldosterone antagonists may be useful in COVID-19, but the ACE2 expression is increased in animal studies using mutant mice.
15)16)17)19) However, human studies reported that ACEI or ARB was not significantly associated with mortality.
15)16) In this study, the use of ACEI or ARB showed no significant relation to clinical severity. The second is myocardial damage due to hypoxia.
5)10)11) The cardiac enzyme of severe patients requiring invasive oxygen therapy is significantly higher than that of mild patients in this study. The last mechanism is the systemic inflammatory response, such as the cytokine storm.
5)11)13) In a cardiovascular pathology study, myocarditis was reported because of increased interstitial macrophages and multifocal lymphocytes,
13) which agrees with what the present study found, where the CRP significantly increased in severe patients compared to mild patients.
A change in QT intervals in SARS-Cov-2 infections can be secondary to the viral infection itself, the inflammatory state of SARS-Cov-2 infection, and ischemia or hypoxia,
7) as shown by how the human immunodeficiency virus and dengue fever have been associated with a prolonged QT interval. Moreover, an animal study using rabbits showed that an acute coronavirus infection was associated with QT interval prolongation. In contrast, systemic inflammation and elevated CRP have been associated with a prolonged QT interval.
4)7)14)20) Farre et al.
6) reported that COVID-19 infection could prolong the QT interval, affecting the heart and causing arrhythmias.
21) The QT interval in an ECG reflects repolarization and can cause cardiac arrhythmia when prolonged. Furthermore, a good prognosis has been reported in patients without an increase in the QT interval.
22) There have also been reports of prolonged QT in patients whose myocarditis was demonstrated by myocardial biopsy.
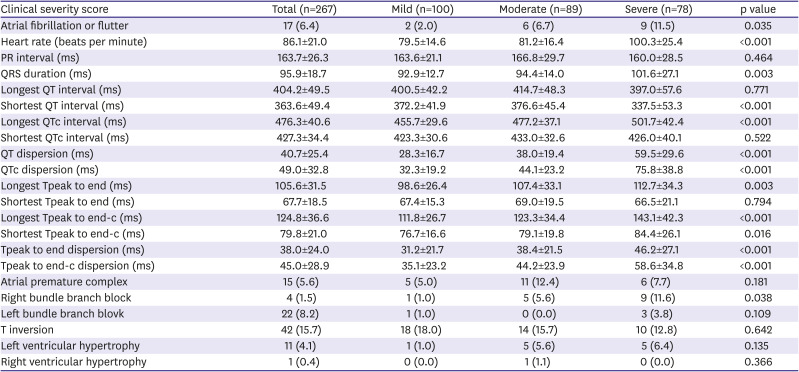
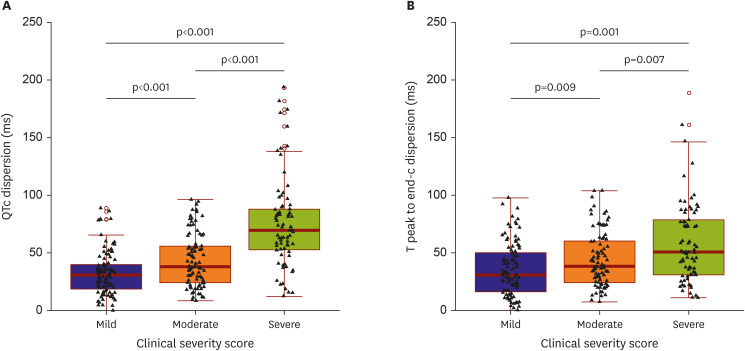
23) Based on this study's results, the longest QT interval was more prolonged as disease severity worsened. In addition, this study evaluated the QT dispersion, which is a simple, approximate evaluation of the overall heterogeneity of ventricular repolarization,
7) and is a surrogate marker for ventricular repolarization.
8)
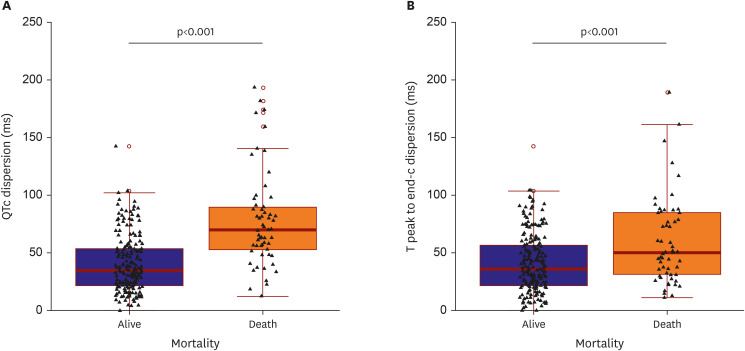
The QTc dispersion of severe patients significantly increased compared to those of mild patients based on the results of the present study. The patients' Tpe was also assessed, another marker of ventricular repolarization dispersion
8)9) that corresponds to the epicardium and myocardium repolarization. However, some cells in the subendothelial tissue are sensitive to early depolarization, possibly leading to arrhythmia. Ozturk et al.
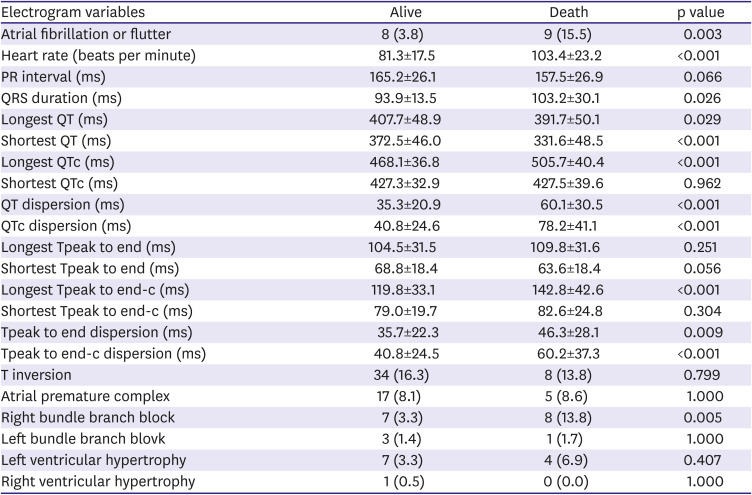
21) demonstrated that the Tpe and QT were higher in COVID-19 patients than in the study’s control group. Similar to the QT interval, Tpe and Tpe dispersion significantly increased in severe patients compared to mild patients in the present study. Therefore, COVID-19 infection could be associated with ventricular repolarization because both QTc and Tpe-c dispersion in severe patients significantly increased compared with mild patients.
Increased QT and QT dispersion have been shown as risk factors for sudden cardiac death caused by cardiac arrhythmias.
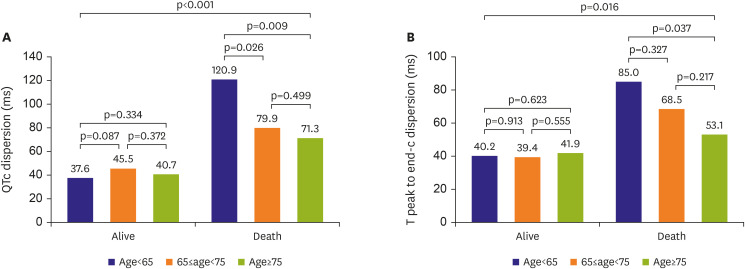
8)9) In terms of QT prolongation in COVID-19, a prolonged QTc interval was associated with higher mortality. Based on the present study's results, deceased patients had prolonged QT intervals compared to surviving patients. Moreover, QTc and Tpe-c dispersion in deceased patients significantly increased compared to those in surviving patients.
There are some limitations to our study. First, this study was a retrospective and observational study. Therefore, we could not determine whether the cause of death in the deceased patients was due to malignant ventricular arrhythmias or if the alive patients suffered from arrhythmias. Second, 267 patients were enrolled to our study although 822 patients admitted at large hospitals due to COVID-19, because the rest of patients could not be underwent ECG test after confirming the COVID-19 due to potential transmission of COVID-19. The most patients were not followed after discharge. Third, the time difference between the onset of COVID-19 and ECG measurement could not be accurately determined which may affect the QT prolongation. While ECG were taken at admission in the most case, laboratory parameters like cardiac enzymes and CRP were obtained at peak level and severity score of patients was at the worst condition. This potential wide spectrum of disease severity is hard to correct statistically them. Fourth, drugs that might affect QT intervals have not been investigated before admission. However, the drugs' effect appeared to be minimal because the ECG was taken at admission.
In conclusion, up to our knowledge, this is first study which shows the association between the COVID-19 infection and cardiac repolarization abnormality by ECG parameters in relatively large Korean patients. COVID-19 infections could significantly prolong QT intervals which stand for the repolarization abnormalities caused by cardiac injuries. Furthermore, the degree of QT interval prolongation might be related to clinical severity by COVID-19 and QTc dispersion of more than 56.1 ms could be an independent predictable factor of mortality.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download