INTRODUCTION
Heart failure (HF) is a global public health problem that affects an estimated 26 million people worldwide,
1) with an increasing prevalence and mortality rate.
2)3) Due to immobility and bedridden status, the short-term risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT), pulmonary embolism (PE), and thromboembolism in other veins, increased in patients with HF
4)5) and may contribute to worse prognoses.
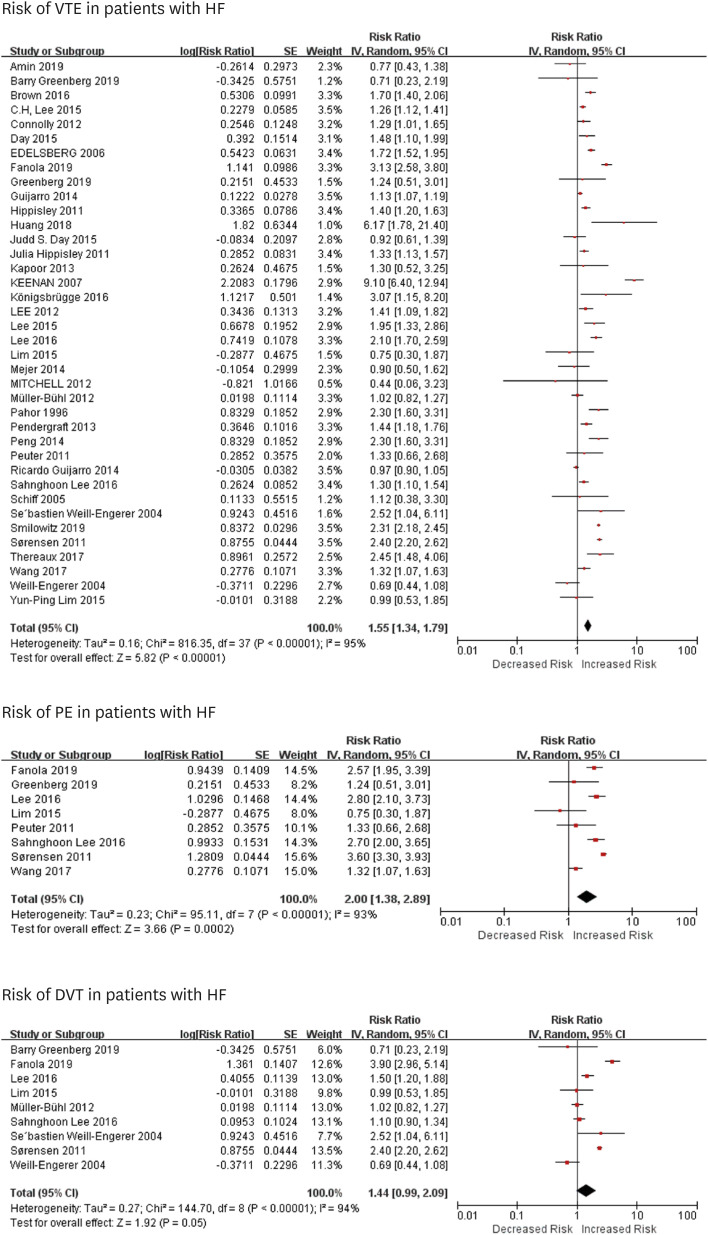
6)7) A previous meta-analysis showed that the in-hospitalized incidence of VTE in patients with HF was 2.66% and the risk of VTE in in-hospitalized HF patients (<60 days) was almost 70% higher compared with non-HF patients.
6) Clinical trials revealed that short-term thromboprophylaxis could be beneficial in HF patients.
7) However, the risk of VTE in out-patients with HF in a long-term period is still controversial,
8)9)10) which leads to unclear recommendations for long-term treatment in HF patients. Given these inconsistencies, we synthesized available data to quantify the risk of VTE in HF in long-term follow-up time.
METHODS
Search strategy
Our search was performed according to the recommendations of the Meta-Analysis of Observational Studies in Epidemiology Group.
11) We searched for records published up to April 15, 2020, in PubMed, MEDLINE, and Embase databases, using keywords “venous thromboembolism” or “vein thromboembolism” or “vein thrombosis” or “venous thrombosis” or “deep venous thrombosis” or “deep vein thromboembolism” or “deep venous thromboembolism” or “deep vein thrombosis” or “pulmonary embolism” or “lung embolism” or “lung thromboembolism” or “pulmonary thromboembolism” and “heart failure” or “cardiac dysfunction” or “cardiac failure” (
Supplementary Table 1). The search was restricted to human studies, but there were no language or publication form restrictions. The strategies for other databases were similar but adapted where necessary. Reference lists were also manually checked to identify other potential studies.
Selection criteria
Cohort studies or post hoc analyses of randomized controlled trials (RCTs) were included in this analysis. Patients who diagnosed HF (chronic HF: with the combination of symptoms [such as dyspnea] and cardiac dysfunction proven by an echocardiogram [left ventricular ejection fraction [LVEF] <50%] or defined by International Classification of Diseases, Ninth Revision [ICD-9] code, acute HF: defined by ICD-9 code) with an over 3-month followed-up time were eligible. We included datum if they presented original data on rates (number of events per follow-up period) or relative risks like risk ratios (RRs), odds ratios, and hazard ratios of all VTE (primary outcome), or DVT or PE alone (secondary outcomes) in HF patients, compared with patients without HF. Diagnoses of VTE, DVT or PE were based on ICD, or imaging examination such as compression venous ultrasound, ventilation-perfusion scan, gray-scale, and Doppler sonography or venography. Animal studies, cross-sectional studies, case-control studies, case reports, studies with follow-up periods <3 months, or data derived from the same studies were excluded. We did not exclude patients with cancer or patients undergoing surgery, who are already at high risk for VTE. Therefore, we were able to include all studies in our meta-analysis and comprehensively investigate the risk of VTE, PE or DVT, and HF in long-term follow-up duration.
Data extraction
Two investigators independently conducted literature searches, reviewed the potential articles, and abstracted data from eligible studies. Discrepancies were adjudicated by discussions with other investigators.
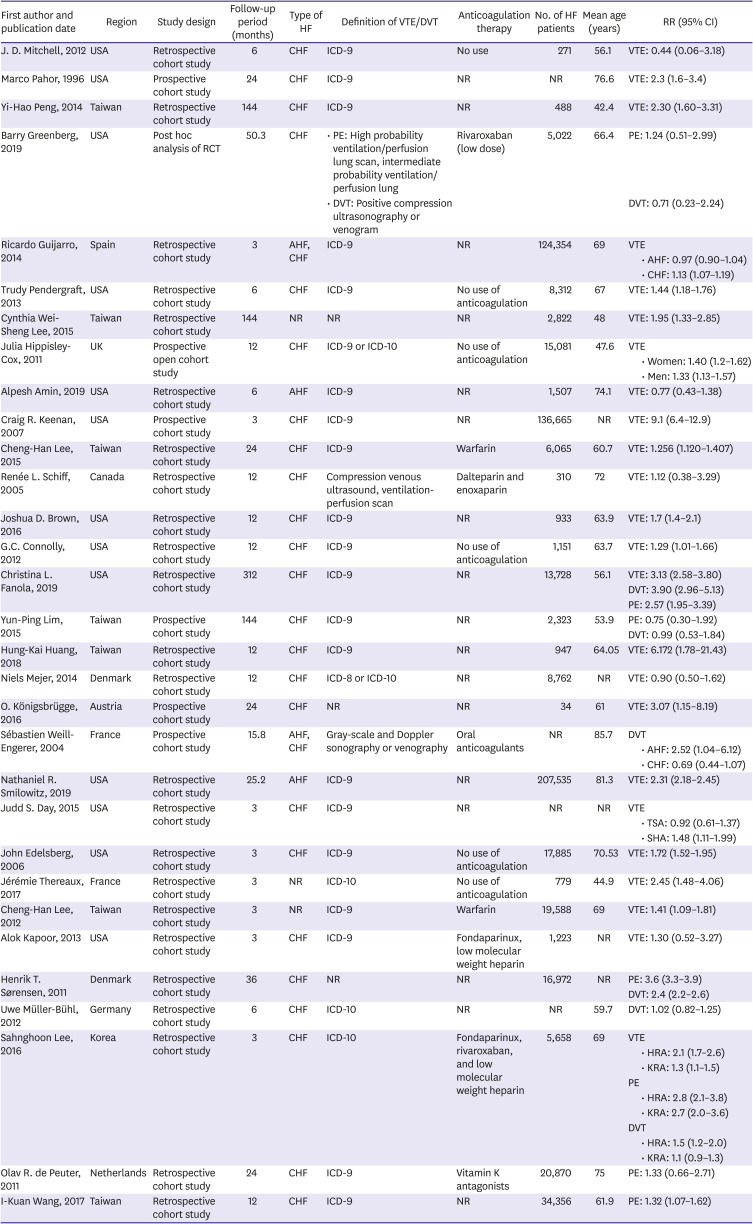
All available data were extracted from included studies, including country or region, study design, patient's population with HF, type of HF, follow-up time, use of anticoagulation therapy, number of participants with HF, ages of participants, adjusted relatives risks with 95% confidence intervals (CIs) and diagnostic criteria for VTE, PE, and DVT.
Quality assessment of studies
We assessed the risk of bias with an adapted and modified Newcastle-Ottawa Scale for observational studies, recommended by previous researchers.
6) There were 6 items and 1 point was scored for each item. The judgments are diagnosis of VTE, PE, and DVT, HF diagnoses, study population (whether are restricted to patients with surgery or cancer), adjustment for age and sex, adjustment for recent major surgery and active malignancy and adjustment for other risk factors. Studies that received one point in all 6 items were judged as high quality (
Supplementary Table 2).
Synthesis and analysis
Relative risks of VTE, PE, or DVT from each study and 95% CIs were logarithmically transformed and the corresponding standard errors (SEs) were calculated to stabilize the variance and normalize the distribution. We used the inverse variance method to combine the calculated log RRs and SEs. We investigated statistical heterogeneity across studies with the I
2 statistic and did sensitivity analyses by omitting one cohort at a time. We assessed publication bias with Begg's test and inspecting funnel plots in which the natural log of RR was plotted against its SE.
12) Meta-regression analysis was used to determine the impact of participants' age and follow-up duration upon the outcomes if data were reported in more than 10 studies according to Cochrane guidelines.
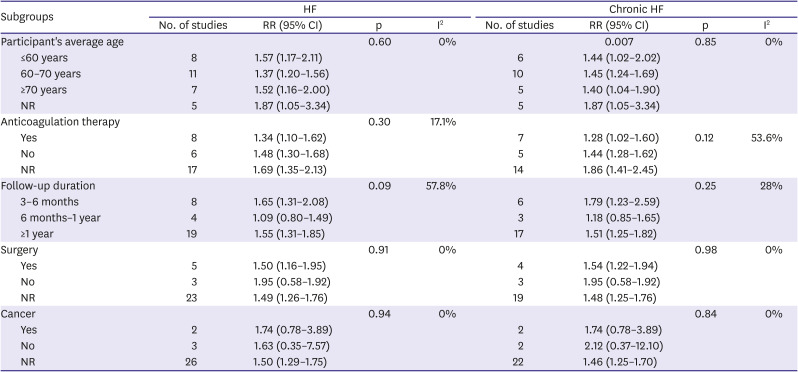
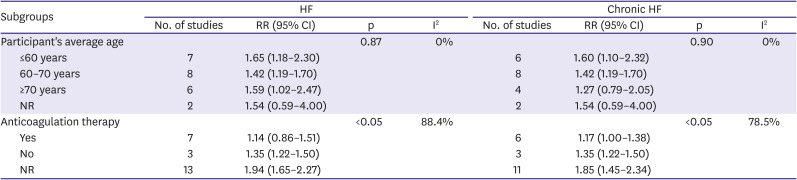
13) We did subgroup analyses of primary outcomes based on study characteristics: type of heart failure (acute, chronic, and not reported), follow-up duration (3–6 months, 6 months–1 year, >1 year), anticoagulation therapy (no anticoagulation therapy, anticoagulation therapy in clinical practice, not reported), age (<60 years old, 60–70 years old, >70 years old), surgery (underwent, not underwent, not mentioned) and, cancer (suffered, not suffered, not mentioned). The p values were 2-tailed, and statistical significance was set at 0.05. All analyses were conducted using RevMan (version 5.3; The Cochrane Collaboration, Copenhagen, Denmark) and Stata software (version 15.0; Stata Corp LP, College Station, TX, USA).
DISCUSSION
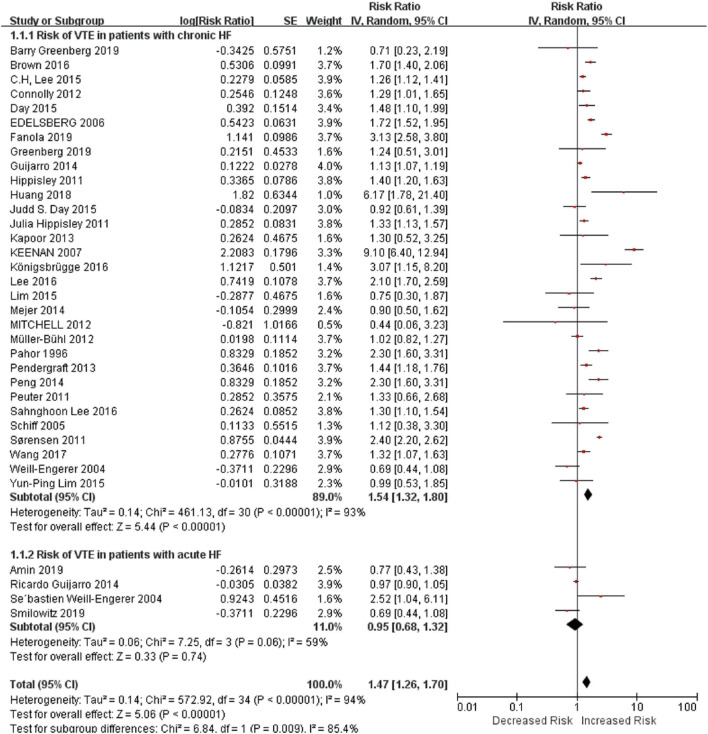
In this study, we noted that HF was an independent risk factor for VTE and PE in long-term follow-up duration, with an RR of about 1.57 and 2.00, respectively, while it was not significant for DVT. Patients with chronic HF had a higher risk of VTE. Subgroup analysis showed that after stratified by follow-up time, anticoagulation therapy, age or surgery, chronic HF was still a risk factor for VTE.
Links between HF and VTE are not well reported. Several mechanisms, such as stasis of blood because of dilatation of cardiac chambers, reduced myocardial contractility and increased intracardiac and central venous pressures, increased viscosity and coagulability of plasma, inflammation, neurohormonal activation, and endothelial dysfunction could contribute to the hypercoagulable state in patients with HF, raising the risk of VTE.
14)
Our findings have several clinical implications. First, we found that HF could be a risk for VTE, and PE, but not for DVT in extended follow-up duration. It may be caused by several reasons. One is that much attention was paid to screen the lower extremity DVT (LEDVT), without realizing DVT in other location such as upper extremity, pelvis, and abdominal organs.
15) Studies showed that upper extremity DVT (UEDVT) had a higher prevalence of HF when compared to patients with LEDVT (20% vs. 6.6%).
16) Hospitalized HF patients often undergo central venous puncture and catheterization to receive therapy, most of which are through the upper extremity such as internal jugular vein, and above 10% of them would develop DVT.
17) Also, some patients with undefined cardiomyopathy or who needed pulmonary artery pressure monitoring often undergo right heart catheterization, which may cause right ventricular thrombus
18) and lead to severe PE.
19) Moreover, patients who were treated with left ventricular assist devices or suffered from cardiomyopathy, like arrhythmogenic right ventricular cardiomyopathy, and dilated cardiomyopathy, were prone to develop right ventricular thrombus.
20)21) Studies demonstrated that the risk of PE was at least twice in patients with HF and the risk increased as LV systolic function declined,
22) especially in those severe decompensated HF patients.
23) These results remind us that we should not neglect the risk of those patients with uncommon venous thrombus especially for those who are frequently rehospitalized, with long-term in-hospitalized stay or receiving catheter therapy. Another explanation is that DVT is hard for some patients to recognize and is often neglected by physicians because it could be asymptomatic. Nonetheless, symptoms of DVT are similar to symptoms of HF,
24) so physicians may mistake them as worsening HF without realizing DVT.
Second, we noted that patients with chronic HF were more prone to have VTE than acute HF in the long-term period. Acute HF consists of new-onset HF, caused by acute primary cardiac dysfunctions (such as acute myocardial dysfunction and acute valve insufficiency), and decompensated chronic HF triggered by precipitant factors like infection.
25) For those patients with new-onset HF, part of them can reverse with timely treatment,
26) thus might not have the risk for VTE. Moreover, acute HF patients who should receive surgery such as myocardial infarction, are often prescribed thromboprophylaxis therapy to prevent thrombotic events,
25)26) which may further reduce the occurrences of VTE. As for chronic HF, different factors are prone to result in different types of HF. An epidemiological study suggested that myocardial infarction more often causes HF with reduced ejection fraction (HFrEF), while atrial fibrillation is likely to cause HF with preserved ejection fraction (HFpEF).
25) However, we still do not have studies on differential effect on VTE depending on HF type.
Third, we identified that the risk for VTE still existed in prolonged follow-up period (above 1 year). It reminds us that we should not ignore the risk of VTE in patients even after discharge from hospitals. Anticoagulation therapy is more prone to be prescribed in-hospitalization
27) as most hospitalized HF patients in hospitalized are immobile and bedridden or are applied central venous puncture and catheterization
17) and few patients will continue to receive anticoagulation therapy after discharge.
28) This study implies that we should focus more on thromboprophylaxis in HF patients to prevent VTE out-of-hospitalization. However, we noticed that anticoagulation therapy could reduce the risk of VTE, but the effect was not significant. Another study showed that low-dose (2.5 mg rivaroxaban) did not reduce the incidence of VTE.
9) It prompts us that a higher dosage and an extended duration might benefit patients with HF.
28)
Our study had several limitations. First, we were unable to analyze the impact of severities and types of chronic HF on VTE to provide more insights into the risk of VTE in sub-group patients, as data (such as level of plasma N-terminal pro-brain natriuretic peptide, LVEF and etiology like atrial fibrillation) were not available in most of the included studies. Second, there were still few studies about the risk of DVT in patients with HF, so our analysis may underestimate the risk of DVT in HF patients. Third, our analysis only included 4 studies about acute HF and only 1 reported the risk of VTE in patients with new-onset HF. Thus, we lack enough data of acute HF patients, especially those new-onset HF patients, to distinguish whether acute HF or chronic HF would have a noticeable effect on the VTE risk. Forth, there was substantial heterogeneity among most analyses due to their different follow-up durations, types of diseases and complications, population, etc. Therefore, there may be large uncertainty of VTE rates overall and in subgroups. Fifth, we was not able to show the associations of activity level and the risks of VTE, PE, and DVT, since the data of included studies was limited. Finally, most of our studies were retrospective studies, and prospective studies with high quality such as RCTs are needed to testify our results.
In conclusion, HF was an independent risk for VTE and PE but not DVT in the long-term follow-up period. Patients with chronic HF were prone to have a higher risk of VTE. Further designated and prospective studies are needed for our better understandings of impacts of HF on the risk of VTE, PE and, DVT. RCTs about anticoagulation therapy in HF patients to prevent VTE, PE, and DVT are also needed for better management of HF patients.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download