INTRODUCTION
Depression commonly occurs in patients with coronary artery disease (CAD), including myocardial infarction (MI) and angina pectoris, with a prevalence of over 20%.
1)2)3) The association between depression and CAD are suggested to be bidirectional; CAD can cause major depressive disorders, and depression is an independent relating factor for CAD and its complications.
4) A depression diagnosis at any time following CAD diagnosis has been associated with a 2-fold higher risk of mortality.
5)
Identifying depression may improve psychological health in CAD survivors. However, depression is under-recognized in CAD patients, as healthcare providers rarely use standardized screening tools in primary care and community settings.
6) In a systemic review of screening tools in detecting depression in CAD patients,
7) the 9-item Patient Health Questionnaire (PHQ-9), the Beck Depression Inventory-II, and the Hospital Anxiety and Depression Scale for Depression were reported to be widely used. Although several well-established screening tools for depression have been developed and are commonly used, there is no consensus on the optimal screening instrument for identifying depression in CAD patients.
7) Among these screening instruments, PHQ-9 is a brief and commonly recommended screening tool for assessing depression in clinical practice and research.
4)8)9) Moreover, PHQ-9 (cut-off value of ≥10 points) has a relatively high specificity and high positive predictive value than other tools,
10)11) which means that no additional process for confirming depression may be required in CAD patients with positive results on PHQ-9 assessment.
7)
Previous studies including one conducted in Korea have examined whether sex difference exists in the prevalence of depression among CAD patients, and their results showed that female patients with CAD had a greater risk for depression than their male counterparts.
1)12) However, few studies have been conducted to evaluate the sex difference in the risk of depression in patients with CAD, compared with those without CAD in a community-dwelling population. Therefore, this study aimed to identify the sex-specific associations between CAD status and the severity of depressive symptoms, using a representative nationwide sample of the Korean population.
METHODS
Ethical statement
The study protocol was approved by the Institutional Review Board of Wonkwang University Hospital (WKUH 2020-05-032).
Study population
This study was based on the data of the Korea National Health and Nutrition Examination Survey (KNHANES). The KNHANES is an ongoing surveillance system that identifies the current health and nutritional status of Koreans, monitors trends in health risk factors and the prevalence of major chronic diseases, and provides data for the development and evaluation of health policies and programs.
13) The KNHANES is a cross-sectional survey with nationally representative samples of the Korean population, which is conducted annually by the Korea Centers for Disease Control and Prevention. The health examination and health interview of the KNHANES are performed first by trained medical staff, laboratory technicians, and interviewers. One week later, nutrition surveys are performed by dieticians, who visit the participants' homes. The PHQ-9 scale, which is an instrument for screening, diagnosing, and measuring of depressive disorder, was used only in 2014, 2016, and 2018 KNHANES. Among 13,462 people aged 40 years and older, 2,691 people without information based on PHQ-9 and/or those with missing data on socioeconomic status, health behaviors, and health status were excluded. In total, 10,771 people (4,620 male and 6,151 female) were included in the final analyses.
Measurements
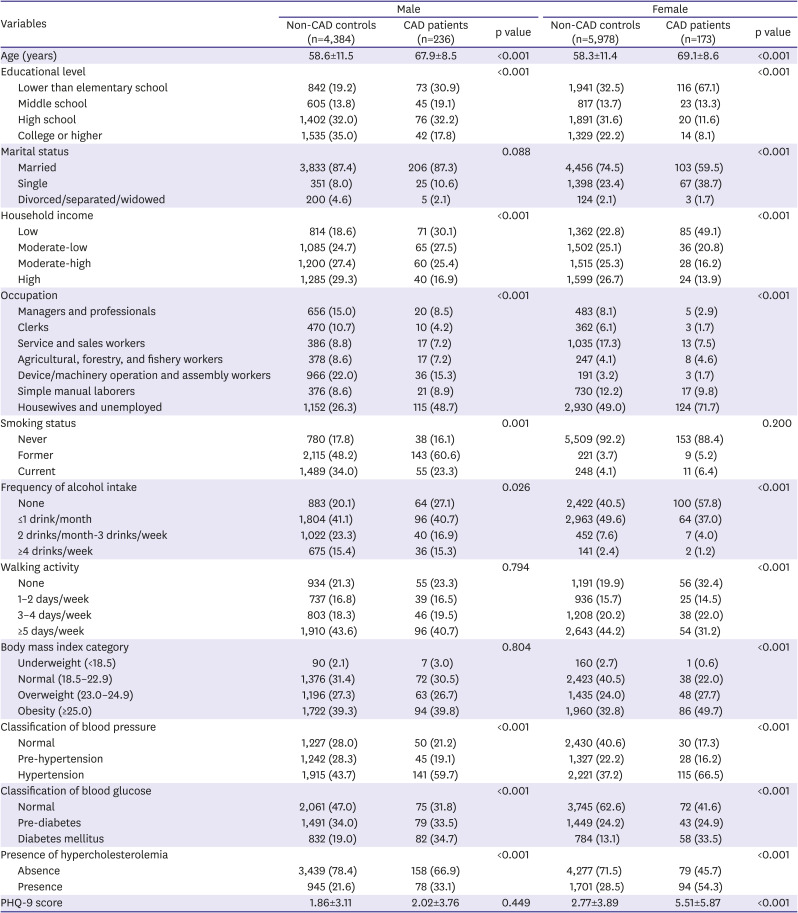
Participants' information regarding demographics, socioeconomic status, health-related behaviors, and medical history was collected from health interviews and examinations. Education level was classified into 4 groups: lower than elementary school, middle school, high school, and college or higher. Marital status was classified into married, single, and others (divorced, separated, and widowed). Household income was calculated by dividing the total household monthly income by the square root of the household size, and the results were grouped into 4 quartiles: low, moderate-low, moderate-high, and high. Occupation was classified into 7 groups: 1) managers and professionals, 2) clerks, 3) service and sales workers, 4) agricultural, forestry, and fishery workers, 5) device/machinery operation and assembly workers, 6) simple manual laborers, and 7) housewives and unemployed. Since socioeconomic status can be a significant confounder, related variables were adjusted in the analysis.
14)
Smoking status was categorized as never, former, and current smoker. The frequency of alcohol intake was grouped into none, ≤1 drink/month, 2 drinks/month to 3 drinks/week, and ≥4 drinks/week. Walking activity was classified into none, 1–2 days/week, 3–4 days/week, and ≥5 days/week based on the number of days of walking for more than 30 minutes a day. Body mass index (BMI) was calculated as body weight divided by the square of height and categorized as underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obesity (≥25.0 kg/m2). Blood pressure (BP), fasting blood glucose (BG), and total cholesterol levels were measured, and current medications for hypertension, diabetes mellitus, and hypercholesterolemia were assessed. Subsequently, BP level was classified into normal (systolic BP <120 mmHg and diastolic BP <80 mmHg), pre-hypertension (systolic BP 120–139 mmHg and diastolic BP 80–89 mmHg), and hypertension (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or currently being treated). BG level was classified into normal (fasting BG <100 mg/dL), pre-diabetes (fasting BG 100–125 mg/dL), and diabetes mellitus (fasting BG ≥126 mg/dL or currently being treated). The presence of hypercholesterolemia was defined as total cholesterol level ≥240 mg/dL or currently being treated.
The PHQ-9 is a reliable and validated instrument for measuring the severity of depressive symptoms and rendering criteria-based diagnoses of depression in primary care.
9) The PHQ-9 contains 9 symptom-related items that measures the frequency of experiencing depressive symptoms over the past 2 weeks. The total PHQ-9 score can range from 0 to 27 points, based on the response options of ‘not at all (0 point),’ ‘several days (1 point),’ ‘more than half the days (2 point),’ and ‘nearly every day (3 point)’ for each item. In this study, the PHQ-9 score was dichotomized as normal (<10 points) or depression (≥10 points), because it showed high sensitivity and specificity for major depression.
9)15)
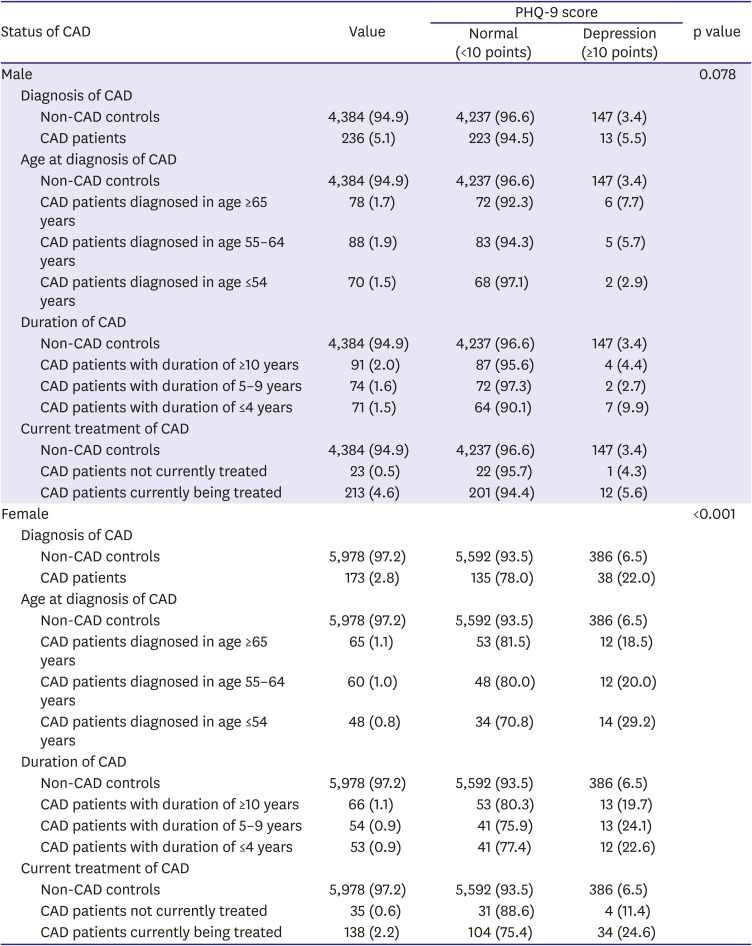
Information on whether the participants had been diagnosed with CAD, including MI or angina pectoris, was obtained from them. Among patients diagnosed with CAD, the age at diagnosis and whether they were currently being treated for CAD was further identified. In addition, the duration of CAD was calculated as the current age minus the age at diagnosis.
Statistical analysis
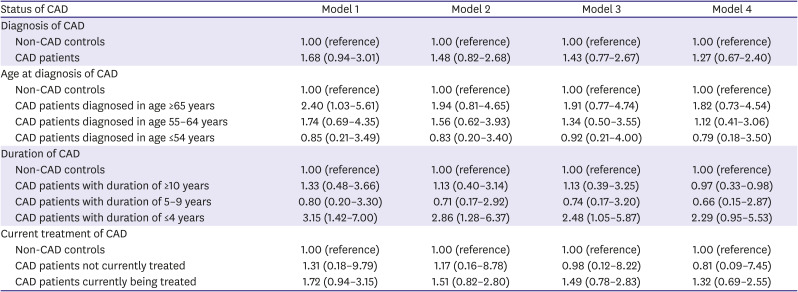
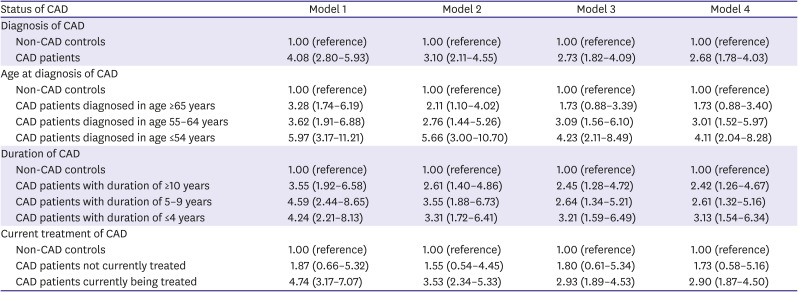
All statistical analyses were performed separately for male and female, because sex and CAD have significant interaction (p<0.05). The characteristics were compared based on the presence or absence of CAD using chi-square test for categorical variables and the Student's t-test for continuous variables. The prevalence rates of depression were compared according to various indications of CAD status (diagnosis of CAD, age at diagnosis of CAD, duration of CAD, and current treatment of CAD) using chi-square test. Multiple logistic regression was used to explore the independent association between CAD status and depression; the odds ratio (OR) and 95% confidence interval (CI) were calculated. Model 1 was unadjusted, while model 2 was adjusted for age. Model 3 was further adjusted for educational level, marital status, household income, occupation, smoking status, drinking frequency, walking activity, and BMI category, plus model 2. Finally, model 4 was further adjusted for classification of BP, classification of BG, and presence of hypercholesterolemia, plus model 3. Data were analyzed using IBM SPSS Version 24.0 (IBM Corp., Armonk NY, USA). A p value less than 0.05 was considered statistically significant.
DISCUSSION
This study identified the sex difference in the association between CAD and depression screened by PHQ-9. Female with CAD had a 2.68-fold higher risk for depression than female without CAD, while no significant association between CAD diagnosis and depression was observed in male. Moreover, only in female, younger age at diagnosis, shorter duration of CAD, and current treatment of CAD may be independent associating factors for depression in this representative nationwide Korean population.
Epidemiological studies have reported higher prevalence of depression in CAD patients of 20% to 60%.
1)2)3) This study reported a prevalence of depression of 12.5%, which is relatively lower than that reported in previous studies. This discrepancy results from differences in the study population, assessment methods and timing, and disease severity. In this study, lower prevalence of depression among CAD survivors in the community might be associated with a relatively lower severity of CAD and a lower frequency of the acute phase, compared with those reported in previous studies predominantly involving in-hospital CAD patients with acute or severe conditions. A previous Korea study using the 2008–2010 KNHANES data reported that the prevalence of depression was 21.7% in adults with CAD aged 30 years and above,
2) which is much higher than that reported in our study. However, that study simply identified depression through 2 questionnaires: whether the participants had ever been diagnosed with depression by a physician and whether they had been sad or depressed for more than 2 weeks in the preceding year, and depression was not confirmed using a validated tool like that used in our study.
2)
A previous meta-analysis of 16 studies showed that depression after MI was associated with 32% higher risk of all-cause mortality and 19% higher risk of cardiovascular events.
16) A recent meta-analysis of 9 cohorts indicated that depression was associated with 76% higher risk of all-cause mortality and 110% higher risk of major adverse cardiovascular events in CAD patients receiving percutaneous coronary intervention.
17) Moreover, both premorbid and postmorbid depression onsets in CAD patients have prognostic significances for adverse cardiac outcomes, showing that the timing of depression may be irrelevant to cardiac mortality and morbidity.
18) Mortality rate was higher even in acute MI patients with mild and clinically insignificant depressive symptoms.
19)
Although the underlying mechanisms for the association between depression and poor clinical outcomes in CAD patients are not fully understood, some factors may be involved in the link between depression and prognosis. First, individuals with depression are more likely to smoke and have higher nicotine dependence and greater difficulty to quit smoking.
20) Second, depression leads to non-adherence to medication in CAD patients,
21) and poor compliance to lipid- and BP-lowering medications in patients at high cardiovascular risk.
22) Third, higher depressive symptoms are associated with lower self-management and higher hemoglobin A1c level, showing that depression is linked to hyperglycemia in people with diabetes.
23) Fourth, depression after MI diagnosis is associated with cardiac autonomic dysfunction (increased heart rate and decreased heart rate variability), which is a strong predictor of mortality in MI patients.
24)
Previous studies have repeatedly shown that female are at a higher risk of depression than male in the general population, and the female to male ratio of the prevalence of depressive disorder is widely accepted at approximately 2:1.
25) A recent meta-analysis of representative samples from 90 countries confirmed that the prevalence of major depression was 2 times higher in female than in male; the sex gap peaked in adolescence and remained stable through adulthood across nations.
26) Similar to the general population, a meta-analysis of CAD patients showed that female with CAD have approximately twice the prevalence of major depression compared to male with CAD.
12) Sex is the most common and strongest factor associated with the prevalence of depression among CAD patients.
2) However, to date, there are insufficient studies that have assessed whether CAD patients have a greater prevalence of depression compared to the general population without CAD, by sex. The present study showed that CAD diagnosis was not a significant predictor for depression in male. On the contrary, female with CAD had a 2.68-fold higher risk for depression than those without CAD.
This study also showed that age at diagnosis and duration of CAD might be independent associating factors for depression among female in a community setting. Moreover, the relationship between age at diagnosis, duration of CAD, and depression was gradual; younger age at diagnosis and shorter duration due to recent diagnosis were significant predictors for depression in female. In this study, there was no significant difference in the prevalence of depression in male of all ages and in female diagnosed after 65 years of age, compared to non-CAD controls in both sexes. However, female diagnosed with CAD at an earlier age of ≤64 years appeared to have a higher prevalence of depression compared with those without CAD. Female diagnosed at a younger age might particularly benefit from aggressive screening and management of post-CAD depression because younger female have a higher prevalence of depression than other sex-age groups among MI patients.
27) Our results are significant considering the findings that depressed younger female with CAD are the group with the greatest risk of cardiovascular events and death, compared with depressed male and older female with CAD.
28)
Screening of all CAD patients for depression should be performed because there is a more convincing evidence showing that depression affects cardiac morbidity and mortality, health behaviors, and treatment compliance.
4) After screening for depression in CAD patients, further diagnosis, appropriate management, and necessary referral may also be required, to improve cardiovascular outcomes.
7) Periodic clinical attention and ongoing public health interests are required to identify and manage CAD patients with depression. Moreover, active treatment of depression is important because untreated depression in CAD patients has been reported to increase mortality risk, compared with CAD patients undergoing treatments for depression or non-depressed patients.
29) A meta-analysis showed that the use of antidepressants such as selective serotonin reuptake inhibitors in patients with CAD and depression, reduces depressive symptoms and improves CAD prognosis.
30)
Several limitations should be discussed. First, since this study is a cross-sectional design, it was difficult to interpret the association as a causal relationship. It was not possible to distinguish whether CAD caused depression or whether depression increased CAD. Second, information about CAD was identified by health interview, but not confirmed by medical records. Recall bias for CAD diagnosis and related detailed data might have been involved. Third, although a valid instrument for assessing depressive disorders was used, depression was not determined by a psychiatrist's medical diagnosis. In addition, since there was no information on the duration of depression, time sequence was not clear. Fourth, only the CAD survivors in the community were included in the survey, while in-hospital patients with acute or severe CAD were not included. Therefore, our findings may have underestimated the effect of CAD status on depression. Fourth, while the present study has shown that CAD diagnosis is not a significant predictor for depression in male, inadequate statistical power may be considered as a reason for this apparent lack of association. Therefore, population studies with much larger sample sizes are needed in the future. Finally, since there is no significant association between CAD and depression in all subjects, in principle, a sub-analysis might not be meaningful. Despite these limitations, this study used a well-designed survey data that was nationally representative of the Korean population. In addition, depression was determined using a standardized assessment instrument for depressive disorders. Detailed information on CAD status including age at diagnosis, duration, and current treatment was analyzed to examine the association with depression by sex.
In conclusion, there is no clearly significant association between CAD and depression in all subjects, but it is necessary to be more careful in female. Therefore, the contribution of sex need to be more intensively considered in the association between CAD status and depression, not only in clinical settings but also in community settings. Consistent screening and management of depression in CAD survivors may improve their quality of life. Designing and implementing psychosocial supports and interventions for promoting mental health of community-dwelling CAD survivors is required, especially for female diagnosed at a younger age and recently diagnosed.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download