INTRODUCTION
The data published by Statistics Korea in 2019 show that pneumonia was third among the 10 most frequent causes of death in 2018 [
1]. A potential cause for such high pneumonia- based mortality among the elderly is the correlation between age and chronic diseases, such as diabetes mellitus, hypertension, chronic obstructive pulmonary disease or cardiovascular disease, which are known to increase one’s risk of developing pneumonia infection [
2]. Previous studies have shown that the risk of invasive pneumonia is increased by three to seven times in patients with underlying diseases [
2,
3].
The main bacterial pathogen of community-acquired pneumonia in Korea is
Streptococcus pneumoniae [
4]. The most frequently observed pneumococcal serotype in Korea between 2008 and 2014 was serotype 3 (13.5%), followed by serotypes 35 (10.8%), 19A (9.0%), 19F (6.6%), and 6A (6.1%). Among them, serotypes 19A and 19F are characterized by multiple resistance to antibiotics, while 6A is the serotype with a particularly high frequency in East-Asia regions [
5]. So, an emphasis has been placed on the prevention for serotypes 19A, 19F, and 6A, and consequently, the need for pneumococcal 13-valent conjugate vaccine (PCV13) that includes all three serotypes 19A, 19F, and 6A, and for pneumococcal polysaccharide vaccine (PPSV23) including serotypes 19A and 19F. Consequently, The Korean Society of Infectious Diseases (KSID) mandates that the patients aged over 65 years with a chronic disease or low-level immune function receive PCV13 vaccination, followed by PPSV23 vaccination a year later, if they had not previously received a PPSV23 vaccination. Similarly, the KSID also recommends that adults between the ages of 18 to 64 or those with low-level immune function should receive the PCV13 vaccination, followed by PPSV23 vaccination a year later [
6]. In Korea, however, the rate of PCV13 vaccination is far lower than that of PPSV23. This is because, as mentioned previously, PPSV23 vaccination is a mandatory vaccination for individuals aged over 65 years in Korea, and moreover, it is free. Therefore, it is necessary to recommend PCV13 vaccination in the high-risk group.
But various factors can affect how a patient decides to be vaccinated. In fact, according to one study, placing a brochure in the office increased the vaccination rate. Giving information about vaccination via telephone and having a poster in the waiting room or examination room helps to improve the vaccination rate. However, among them, the most important thing in the end was clinical reminder or education [
7]. Health care provider’s recommendation is known to have a significant impact on patient decision making [
8]. However, some doctors may find it difficult to make recommendations even when they know the need for vaccination [
9]. Actually, they may not know what to convey and how to make recommendations. According to one study, there are three domains in which patients are reluctant to be immunized: 1) contextual influences, 2) individual/social group influences, and 3) vaccine and vaccination-specific issues [
10]. The main reason for the vaccine hesitancy was risk-benefit based on scientific evidence. The second reason was the individual's knowledge and awareness of the vaccine [
10]. Therefore, it would be important if the recommendations of the physician were made in the clinic based on these contents. But there are also few studies on how best to recommend to patients in a way. So, this study aimed to investigate the effects of the recommendation method on the coverage rate of PCV13 vaccination in high-risk patients upon their first outpatient visit.
METHODS
1. Subjects
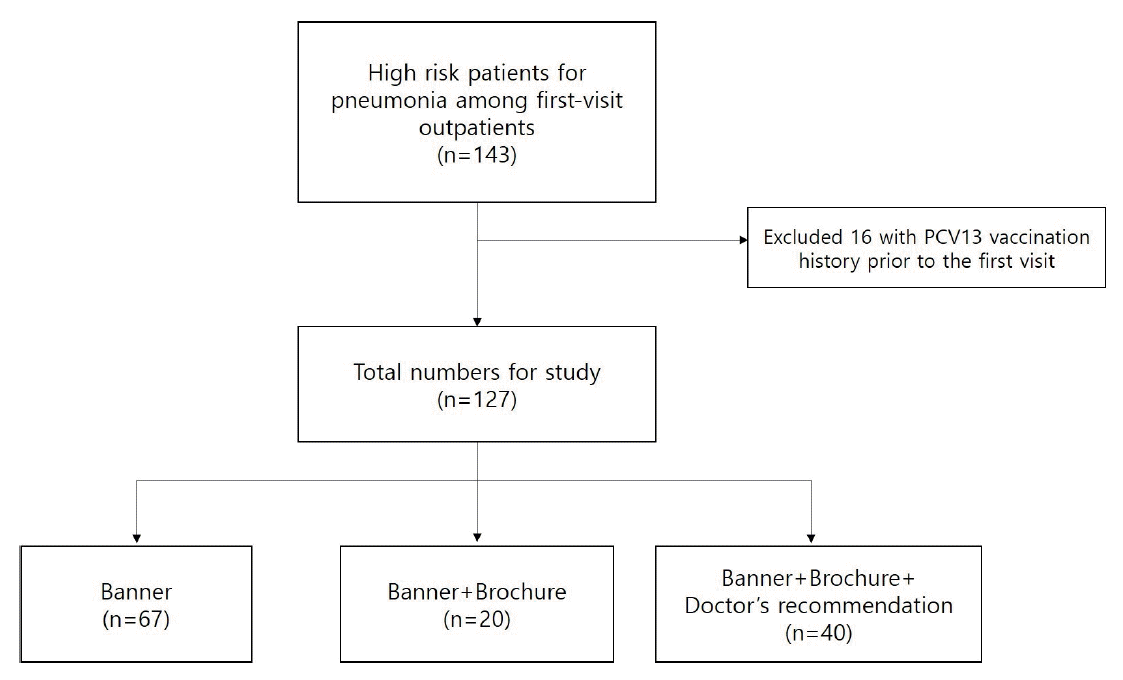
Among the patients who first visited the primary medical institution between March 2019 and February 2020, 143 patients were screened as being at high risk for pneumonia. Excluding 16 patients who were confirmed through the integrated vaccination management system to have received PCV 13 before the visit, 127 individuals were finally selected as the study subjects.
The reason for targeting the first visit patients was to ensure that the recommended strength was constant in the doctor-patient relationship. High-risk patients for pneumonia are defined by the U.S. Advisory Committee on Immunization Practices as those aged over 65 years; under care for diabetes mellitus; with chronic lung disease, cardiovascular disease, liver disease, or kidney disease; diagnosed with and under care for cancer; received solid organ transplantation; received stem cell transplantation; administered with an immunosuppressant; showing asplenia or HIV infection; a chronic smoker; or an alcoholic [
11]. As the subjects of this study did not satisfy all the high-risk criteria for pneumonia, they were divided into three high-risk groups as follows: 1) those who aged over 65 years with or without chronic disease, 2) subjects with chronic diseases such as diabetes mellitus, chronic lung disease, cardiovascular disease, chronic liver disease, chronic kidney disease, diseases on immunosuppressants, or chronic smoking, and 3) malignancy. Because none of the subjects had received solid organ or stem cell transplantation or were alcoholics, these criteria were not included in our study.
The final 127 subjects were also divided into the other three groups according to the vaccination recommendation methods used during counseling: banner (B) group (67 subjects), banner+brochure (B+Br) group (20 subjects), and banner+brochure+doctor's verbal recommendation (B+Br+DR) group (40 subjects). Recommendation methods differed among patients irrespective of high-risk features for pneumonia but according to clinical situation and available time of counseling (
Figure 1).
Figure 1.
Study population and divided into recommendation method. PCV13, 13-valent conjugate vaccine.

2. Study ethics approval
This study was a retrospective cohort study. It was performed in compliance with the Declaration of Helsinki and was approved waiver of informed consent to subjects by the Ethics Committee of Chungnam National University Hospital (Institutional Review Board Number: 2020-02-052-001).
3. Vaccination recommendation
PCV13 vaccination is defined as the completion of vaccination within 1 month from the doctor's recommendation. The date when the recommendation was given and the date of vaccination were recorded in the patient's medical record, which was analyzed in a retrospective manner. In the case of patients who couldn’t confirm their vaccination status, was confirmed through the integrated vaccination management system known as “The Immunization Registry”.
The recommendation methods for pneumonia vaccination used in this study were included the use of a banner, brochure, and doctor's verbal recommendation. The applied method of recommendation was also recorded in the patients’ medical record. A big banner of 60×181 cm in size that describes the need for PCV13 vaccination in high-risk patients for pneumonia was stationed in the waiting room of the clinic (
Supplementary Figure 1A). On the desk inside the office, a small banner of 15×30 cm size with an identical description was placed so as to allow all high-risk patients to be adequately exposed to the information (
Supplementary Figure 1B). Subsequently, patients who were not given any other intervention were categorized as the B group. The patients who were given, in addition to the banner, a brochure explaining the need for PCV13 vaccination based on each high-risk feature for pneumonia were categorized as the B+Br group. The brochure was of 31.3×20 cm size and its contents included the pneumonia mortality rate and a comparison of pneumonia incidence based on each high-risk feature. The brochure was provided to each patient according to the following characteristics: aged over 65, diabetes mellitus, chronic lung disease, chronic cardiovascular disease, immunocompromised, and cancer (
Supplementary Figure 2). The B+Br+DR group patients were given a brochure and a short, one-sentence recommendation regarding the importance of PCV13 vaccination. Here, to minimize the difference in the level of recommendation between doctors, a doctor who have participated this study, not recommended by other department’s doctors, made the recommendation using a phrase resembling "You are a high-risk patient for pneumonia and require PCV13 vaccination", and nonverbal expressions were analogous.
4. Description of other variables
To account for the participants occupation, the 7th Edition of the Korean Standard Classification of Occupations from the Statistics Korea was used, and the patients were categorized into nine occupation groups [
12]. These groups were further divided into manual workers, service/sales workers, non-manual workers, and none to account for the type of physical labor. Specifically, manual workers included simple laborers, technicians or relevant technical workers, mechanics for the manipulation and fabrication of devices and machines, and the workers of agriculture, forestry, or fishery. The service/sale workers included service workers and sales workers, while the non-manual workers included office workers, specialists or relevant special field workers, and managerial workers. If the subject did not belong to any of these categories, he or she was assigned none for occupation.
5. Statistics
In this study, patients aged over 65 years, and those with chronic disease, or cancer were categorized as high-risk for pneumonia. Specifically, patients aged over 65 years were defined as adults not receiving treatments for a chronic disease or cancer; patients with chronic disease were defined as those under care for diabetes mellitus, or those with chronic lung disease, chronic cardiovascular disease, chronic kidney disease, or chronic liver disease, or those administered with an immunosuppressant; finally, patients with cancer were defined as being under care for cancer.
The B, B+Br, and B+Br+DR groups, were compared based on the following characteristics: age, sex, high-risk for pneumonia, PPSV23 vaccination history, occupation and residence. A one-way analysis of variance was used for continuous variables, while a chi-square test was used for analysis of categorical variables (
Table 1).
Table 1.
Baseline characteristics of study subjects by vaccination needs and intervention methods
|
Total (n=127) |
Intervention method
|
|
B (n=67) |
B+Br (n=20) |
B+Br+DR (n=40) |
P
|
|
Age, y |
59.0±14.3 |
59.3±16.3 |
60.7±11.6 |
57.6±11.9 |
|
|
Sex |
|
|
|
|
0.484 |
|
|
Male |
60 (47.2) |
35 (52.2) |
8 (40.0) |
17 (42.5) |
|
|
Female |
67 (52.8) |
32 (47.8) |
12 (60.0) |
23 (57.5) |
|
|
High risk group |
|
|
|
|
0.016 |
|
Aged over 65 years |
29 (22.8) |
22 (32.8) |
2 (10.0) |
5 (12.5) |
|
|
Chronic disease |
65 (51.2) |
34 (50.7) |
12 (60.0) |
19 (47.5) |
|
|
|
Diabetes mellitus |
23 |
9 |
7 |
7 |
|
|
|
Chronic lung disease, chronic smoker |
23 |
16 |
3 |
4 |
|
|
|
Cardiovascular disease |
9 |
3 |
1 |
5 |
|
|
|
Chronic liver disease |
5 |
4 |
- |
1 |
|
|
|
Chronic kidney disease |
1 |
1 |
- |
- |
|
|
|
Use for immunosuppressant |
4 |
1 |
1 |
2 |
|
|
Malignancy |
33 (26.0) |
11 (16.5) |
6 (30.0) |
16 (40.0) |
|
|
Prior vaccination (PPSV23) |
32 (25.2) |
19 (28.4) |
6 (30.0) |
7 (17.5) |
0.395 |
|
Occupation |
|
|
|
|
0.764a
|
|
Manual workers |
53 (41.7) |
27 (40.3) |
10 (50.0) |
16 (40.0) |
|
|
Service/sales workers |
26 (20.5) |
16 (23.9) |
4 (20.0) |
6 (15.0) |
|
|
Non-manual workers |
39 (30.7) |
18 (26.9) |
5 (25.0) |
16 (40.0) |
|
|
None |
9 (7.1) |
6 (8.9) |
1 (5.0) |
2 (5.0) |
|
|
Residence |
|
|
|
|
0.855a
|
|
Urban |
121 (95.3) |
63 (94.0) |
19 (95.0) |
39 (97.5) |
|
|
Rural |
6 (4.7) |
4 (6.0) |
1 (5.0) |
1 (2.5) |
|

In addition, the difference in PCV13 vaccination according to the recommendation method was also analyzed using the chi-square test (
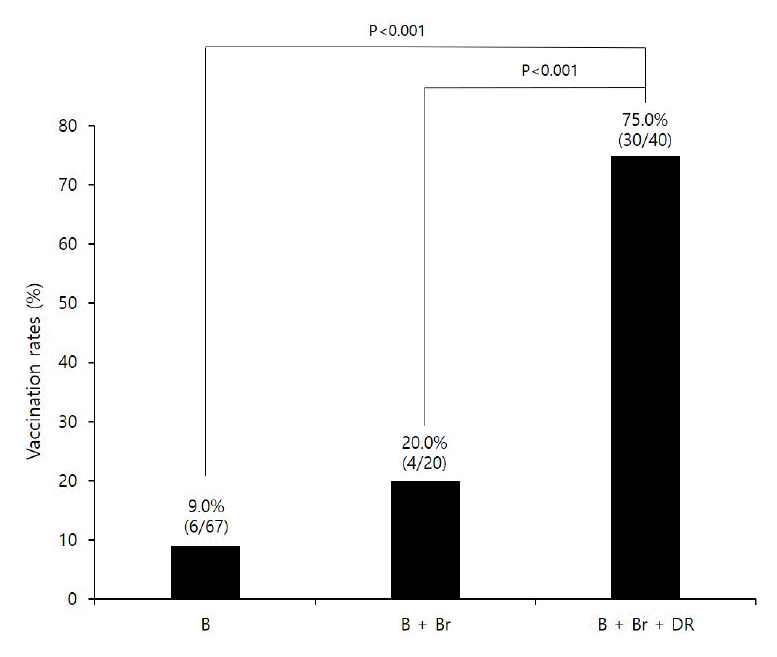
Figure 2).
Figure 2.
Vaccination rates by intervention method among patients with vaccination needs. P-values were represented by chi-square test. B, banner; Br, brochure; DR, doctor’s verbal recommendation.

For the difference in recommendation method between groups who did and did not receive PCV13 vaccination, based on each risk feature regarding pneumonia vaccination, both chi-square test and Fisher’s exact test were used (
Figure 3).
Figure 3.
Recommendation methods (%) among PCV13 vaccinated patients in each risk group. B, banner; Br, brochure; DR, doctor’s verbal recommendation; PCV13, 13-valent conjugate vaccine. *Represents P-value <0.001 compared to non-vaccination group by chi-square test. †,‡Represent P-value 0.001, 0.004 compared to non-vaccination group by Fisher’s exact test, respectively


To compare the odds ratio (OR) of the vaccination rate depending on the recommendation method, binary logistic regression analysis was used. For the analysis, patient data was adjusted for age, sex, high-risk features, occupation, and residence (
Table 2).
Table 2.
OR of vaccination coverage rates compared to banner intervention
|
Intervention method |
OR (95% CI)a
|
|
Banner |
Reference |
|
Banner+brochure |
2.49 (0.55-11.34) |
|
Banner+brochure+doctor’s verbal recommendation |
43.72 (11.52-165.96) |

For all statistical analyses, the IBM SPSS ver. 21.0 (IBM Corp., Chicago, IL, USA) was used.
DISCUSSION
This study investigated the effects of the recommendation method used on the PCV13 vaccination rate in high-risk pneumonia patients after their first outpatient visit. The findings indicated that the patients who received a combination of all three methods, including the doctor's verbal recommendation, resulted in about 43 times higher rate of vaccination in comparison to patients who only received the banner recommendation.
To improve the overall vaccination rate, influences from various factors should be considered. Among them, several studies suggest that the doctor's recommendation is crucial. According to a previous study for influenza vaccination rates in adults aged over 18 years, the doctor's recommendation increased the vaccination rate compared to a lack of recommendation (66% vs. 32%) [
13]. In another study, a doctor’s recommendation was deemed as a significant factor in increasing the influenza vaccination rate in elderly populations, with an observed 2.2 times rate increase [
14]. However, compared to these studies, our study finding showed higher OR, which may be explained by limitations in study design. Socio-economic factors such as marital status, education level, household income, and personal belief in vaccine effectiveness and safety may affect the vaccination rate but were not considered in our study. Another reason for the high OR for vaccination coverage may be because the subject of this study was designed for a high-risk group for pneumonia.
In a study with a different perspective, factors affecting human papilloma virus vaccination rates were examined in the USA and the most significant reason for not receiving the vaccination was found to be the lack of doctor's recommendation [
15].
Furthermore, when PCV13 vaccination rates were investigated after categorizing patients into those aged over 65 years, having a chronic disease, or cancer, the highest rate of vaccination was observed in patients who were given a doctor's recommendation, with values of 80%, 68.2%, and 84.6%, respectively, in each patient group. Across all groups, the vaccination rate was the highest for those who received the doctor's recommendation, irrespective of high-risk features. The results of other studies lend support to this finding. For example, factors influencing pneumonia vaccination in elderly populations were examined in Japan, and the doctor's recommendation was found to exert the strongest influence (8.42 times) on the vaccination rate [
16]. The study also reported that a doctor's recommendation in a primary care setting increased the vaccination rate by approximately 2-4 times for influenza vaccinations in patients with a chronic disease [
17]. In France, factors influencing pneumonia vaccination in patients diagnosed with cancer were examined, irrespective of the use of anticancer therapy, and the highest vaccination rate (12.9 times) was shown by the patients for whom the family doctor had provided vaccination information [
18].
In the case of patients with PPSV23 vaccination history prior to the first visit (data not shown), not a single patient from the B, or the B+Br group received the vaccination. However, the B+Br+DR group showed that, among the seven patients with previous PPSV23 vaccination, six patients (85.7%) received the vaccination (
Supplementary Table 1). This result can be inferred carefully to the fact that, unless the doctor explains the importance of PCV13 and the need for as additional pneumonia vaccination, patients would assume they did not need another vaccination, PCV13. This is supported by a study conducted in Korea where patient surveys reveal common reasons for choosing not to receiving a pneumonia vaccination, that include "I didn't know much about the pneumonia vaccination" and "I wasn't given any recommendation from the doctor" with percentages of 75.9% and 27.8%, respectively [
19], which implies that over 90% of the time, the cause is a lack of information.
It is thought that the provision of the brochure will have an effect on improving the vaccination rate, and previous studies have confirmed that it is also effective. One study found that the group who received the brochure improved the inoculation rate by about 25% or more when comparing the influenza vaccination rate during pregnancy compared to the group that did not [
20]. However, in this study, the provision of banners and brochures did not significantly increase the vaccination rate. There may be various reasons for this, but the important reason is that it is thought that it would have been difficult to convey information to patients only by providing a brochure. According to one study, only 38% of people who were offered a brochure read the brochure, and they thought that providing a single brochure was not enough to convey information [
21]. Therefore, simply providing a brochure may be difficult to deliver sufficient information to the patient. Therefore, making the contents of the brochure interesting to the patient and the delivery method of the brochure are expected to be important and should be applied to future research.
Our findings indicate that, for PCV13 vaccination, a doctor's verbal recommendation is highly significant. Nevertheless, for the cancer patients which is a high-risk feature for pneumonia, anticancer therapy may induce a temporary low state of immunity in patients, such that effectiveness of the vaccination may be reduced, and consequently a doctor would hesitate to recommend vaccination. However, a recent study conducted in Korea evaluating antibody formation in cancer patients vaccinated with PCV13 2 weeks prior to, or on the first day of anticancer treatment. Both cases revealed more than 4-fold increase in antibody formation irrespective of the time of vaccine administration in relation to anticancer treatment [
22]. Excellent stability and immunogenicity were also observed in pediatric patients; among the pediatric patients diagnosed with cancer, PCV13 vaccination was given to those currently under anticancer therapy or within 12 months of termination of therapy, and the findings indicated more than 70% antibody formation in both cases [
23]. Thus, although further studies are warranted, there seems to be no basis for the hesitation in recommending vaccination to cancer patients.
For infants and children in Korea, PCV13 vaccination has been made mandatory by the government in free based on the awareness of complications such as acute otitis media and meningitis caused by Streptococcus pneumoniae. For adults, however, PPSV23 is free of charge only for individuals aged over 65 years. Thus, along with a need for improved institutional support, it is essential that doctors more actively recommend PCV13 vaccination to high-risk patients so that an adequate level of immunity for pneumonia among Korean can be achieved, such the incidence and associated complications, including mortality, can be reduced.
The present study had several limitations. The main limitation of this study is that the total number of subjects was too small because it was performed in a single medical clinic and only subjects in the high-risk group for pneumonia among the first visit, so there were differences in baseline between the three groups. In particular, the B+Br group had a very small number of subjects as it was a primary medical institution and the patients of chronic disease accounted for nearly 50% of the characteristics of high-risk pneumonia groups, making it difficult to compare by disease. In addition, it was difficult to perform subgroup analysis because the total number of subjects was small. Second, the socio- economic factors such as marital status, education level, household income, and personal belief in vaccine effectiveness and safety affecting the coverage rate are missing. Third, since the vaccination period was set to one month after the doctor's verbal recommendations, the vaccination history has not been investigated after the one-month period. Fourth, it was difficult to compare the recommended individual methods. In the future, further research is required to support the results of this study, with more systematic study design considering socio-economic factors of the subjects which may affect the vaccination rate.
Nevertheless, the most valuable findings in this study are significant as they reveal the importance of a doctor's verbal recommendation in the coverage rate of PCV13 vaccination in high-risk pneumonia patients. According to one study, the patient’s and the doctor’s attitude toward vaccination is related to the vaccination rate [
24]. So the doctor recognize the need for PCV13 vaccination in high-risk patients of pneumonia and verbal recommend the message that the patient needs it. Therefore, in patient education, we think that there is a need for doctor’s verbal recommendation from a physician, along with other methods.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download