1. Conte E, Bresciani E, Rizzi L, Cappellari O, De Luca A, Torsello A, Liantonio A. 2020; Cisplatin-induced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci. 21:1242. DOI:
10.3390/ijms21041242. PMID:
32069876. PMCID:
PMC7072891.

2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. 2011; Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12:489–495. DOI:
10.1016/S1470-2045(10)70218-7. PMID:
21296615.

3. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999; Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868. DOI:
10.1016/S0092-8674(00)80595-4. PMID:
10102273.

4. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. 2007; FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6:458–471. DOI:
10.1016/j.cmet.2007.11.001. PMID:
18054315.

5. Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, et al. 2017; Molecular definitions of autophagy and related processes. EMBO J. 36:1811–1836. DOI:
10.15252/embj.201796697. PMID:
28596378. PMCID:
PMC5494474.

6. Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP. 2004; Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology. 145:4806–4812. DOI:
10.1210/en.2004-0406. PMID:
15284206.

7. Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. 2006; Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat
Tibialis Anterior muscle. Mech Ageing Dev. 127:794–801. DOI:
10.1016/j.mad.2006.07.005. PMID:
16949134.
8. Bresciani E, Rizzi L, Molteni L, Ravelli M, Liantonio A, Ben Haj Salah K, Fehrentz JA, Martinez J, Omeljaniuk RJ, Biagini G, Locatelli V, Torsello A. 2017; JMV2894, a novel growth hormone secretagogue, accelerates body mass recovery in an experimental model of cachexia. Endocrine. 58:106–114. DOI:
10.1007/s12020-016-1184-2. PMID:
27896546.

9. Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, Ben Haj Salah K, Fehrentz JA, Martinez J, Giustino A, Mariggiò MA, Coluccia M, Tricarico D, Lograno MD, De Luca A, Torsello A, et al. 2017; Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. J Cachexia Sarcopenia Muscle. 8:386–404. DOI:
10.1002/jcsm.12185. PMID:
28294567. PMCID:
PMC5703021.

10. Fanzani A, Zanola A, Rovetta F, Rossi S, Aleo MF. 2011; Cisplatin triggers atrophy of skeletal C2C12 myotubes via impairment of Akt signalling pathway and subsequent increment activity of proteasome and autophagy systems. Toxicol Appl Pharmacol. 250:312–321. DOI:
10.1016/j.taap.2010.11.003. PMID:
21074548.

11. Lee K, Ochi E, Song H, Nakazato K. 2015; Activation of AMP-activated protein kinase induce expression of FoxO1, FoxO3a, and myostatin after exercise-induced muscle damage. Biochem Biophys Res Commun. 466:289–294. DOI:
10.1016/j.bbrc.2015.08.126. PMID:
26342801.

12. Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, Li X, Qin ZH. 2013; Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol. 48:427–436. DOI:
10.1016/j.exger.2013.02.009. PMID:
23419688.

13. Hojman P, Fjelbye J, Zerahn B, Christensen JF, Dethlefsen C, Lonkvist CK, Brandt C, Gissel H, Pedersen BK, Gehl J. 2014; Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLoS One. 9:e109030. DOI:
10.1371/journal.pone.0109030. PMID:
25268807. PMCID:
PMC4182656.

14. Miyagi MY, Seelaender M, Castoldi A, de Almeida DC, Bacurau AV, Andrade-Oliveira V, Enjiu LM, Pisciottano M, Hayashida CY, Hiyane MI, Brum PC, Camara NO, Amano MT. 2014; Long-term aerobic exercise protects against cisplatin-induced nephrotoxicity by modulating the expression of IL-6 and HO-1. PLoS One. 9:e108543. DOI:
10.1371/journal.pone.0108543. PMID:
25272046. PMCID:
PMC4182716.

15. Sirago G, Conte E, Fracasso F, Cormio A, Fehrentz JA, Martinez J, Musicco C, Camerino GM, Fonzino A, Rizzi L, Torsello A, Lezza AMS, Liantonio A, Cantatore P, Pesce V. 2017; Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci Rep. 7:13017. DOI:
10.1038/s41598-017-13504-y. PMID:
29026190. PMCID:
PMC5638899.

16. Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S, Liu L. 2016; The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 50:339–345. DOI:
10.1136/bjsports-2015-094927. PMID:
26385207.

18. Fernández de Mattos S, Villalonga P, Clardy J, Lam EW. 2008; FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther. 7:3237–3246. DOI:
10.1158/1535-7163.MCT-08-0398. PMID:
18852127. PMCID:
PMC2748241.

19. Lu M, Chen X, Xiao J, Xiang J, Yang L, Chen D. 2018; FOXO3a reverses the cisplatin resistance in ovarian cancer. Arch Med Res. 49:84–88. DOI:
10.1016/j.arcmed.2018.04.014. PMID:
29716743.

20. Rashtchizadeh N, Argani H, Ghorbanihaghjo A, Sanajou D, Hosseini V, Dastmalchi S, Nazari Soltan Ahmad S. 2019; AMPK: a promising molecular target for combating cisplatin toxicities. Biochem Pharmacol. 163:94–100. DOI:
10.1016/j.bcp.2019.02.006. PMID:
30738797.

21. Zeng Z, Liang J, Wu L, Zhang H, Lv J, Chen N. 2020; Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front Physiol. 11:583478. DOI:
10.3389/fphys.2020.583478. PMID:
33224037. PMCID:
PMC7667253.

22. Sun M, Huang C, Wang C, Zheng J, Zhang P, Xu Y, Chen H, Shen W. 2013; Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. Biochem Biophys Res Commun. 441:169–174. DOI:
10.1016/j.bbrc.2013.10.039. PMID:
24140059.

23. Kim HJ, So B, Choi M, Kang D, Song W. 2015; Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp Gerontol. 70:11–17. DOI:
10.1016/j.exger.2015.07.006. PMID:
26183690.

24. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. 2004; Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117:399–412. DOI:
10.1016/S0092-8674(04)00400-3. PMID:
15109499. PMCID:
PMC3619734.

26. Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H. 2018; Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol Rep. 6:e13651. DOI:
10.14814/phy2.13651. PMID:
29626392. PMCID:
PMC5889490.

27. Sakai H, Kimura M, Isa Y, Yabe S, Maruyama A, Tsuruno Y, Kai Y, Sato F, Yumoto T, Chiba Y, Narita M. 2017; Effect of acute treadmill exercise on cisplatin-induced muscle atrophy in the mouse. Pflugers Arch. 469:1495–1505. DOI:
10.1007/s00424-017-2045-4. PMID:
28762162.

28. Stefanetti RJ, Lamon S, Wallace M, Vendelbo MH, Russell AP, Vissing K. 2015; Regulation of ubiquitin proteasome pathway molecular markers in response to endurance and resistance exercise and training. Pflugers Arch. 467:1523–1537. DOI:
10.1007/s00424-014-1587-y. PMID:
25104573.

30. Zhao J, Brault JJ, Schild A, Goldberg AL. 2008; Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 4:378–380. DOI:
10.4161/auto.5633. PMID:
18227643.

31. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. 2007; FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6:472–483. DOI:
10.1016/j.cmet.2007.11.004. PMID:
18054316.

32. van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, Verhagen LP, Groot Koerkamp MJ, Braat AK, Dansen TB, Holstege FC, Gebhardt R, Burgering BM, Coffer PJ. 2012; Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat Cell Biol. 14:829–837. DOI:
10.1038/ncb2536. PMID:
22820375.

33. Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. 2015; Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 6:6670. DOI:
10.1038/ncomms7670. PMID:
25858807. PMCID:
PMC4403316.

34. Christensen JF, Jones LW, Tolver A, Jørgensen LW, Andersen JL, Adamsen L, Højman P, Nielsen RH, Rørth M, Daugaard G. 2014; Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. Br J Cancer. 111:8–16. DOI:
10.1038/bjc.2014.273. PMID:
24867693. PMCID:
PMC4090736.

35. Saran U, Guarino M, Rodríguez S, Simillion C, Montani M, Foti M, Humar B, St-Pierre MV, Dufour JF. 2018; Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol Commun. 2:607–620. DOI:
10.1002/hep4.1159. PMID:
29761175. PMCID:
PMC5944574.

36. White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. 2013; Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 304:E1042–E1052. DOI:
10.1152/ajpendo.00410.2012. PMID:
23531613. PMCID:
PMC3651620.

38. Salomão EM, Toneto AT, Silva GO, Gomes-Marcondes MC. 2010; Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Cancer. 62:1095–1104. DOI:
10.1080/01635581.2010.492082. PMID:
21058197.

39. Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, Moresi V. 2016; Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep. 6:26991. DOI:
10.1038/srep26991. PMID:
27244599. PMCID:
PMC4886631.

40. Aversa Z, Pin F, Lucia S, Penna F, Verzaro R, Fazi M, Colasante G, Tirone A, Rossi Fanelli F, Ramaccini C, Costelli P, Muscaritoli M. 2016; Autophagy is induced in the skeletal muscle of cachectic cancer patients. Sci Rep. 6:30340. DOI:
10.1038/srep30340. PMID:
27459917. PMCID:
PMC4962093.

41. Møller AB, Lønbro S, Farup J, Voss TS, Rittig N, Wang J, Højris I, Mikkelsen UR, Jessen N. 2019; Molecular and cellular adaptations to exercise training in skeletal muscle from cancer patients treated with chemotherapy. J Cancer Res Clin Oncol. 145:1449–1460. DOI:
10.1007/s00432-019-02911-5. PMID:
30968255.

42. Toth MJ, Callahan DM, Miller MS, Tourville TW, Hackett SB, Couch ME, Dittus K. 2016; Skeletal muscle fiber size and fiber type distribution in human cancer: effects of weight loss and relationship to physical function. Clin Nutr. 35:1359–1365. DOI:
10.1016/j.clnu.2016.02.016. PMID:
27010836. PMCID:
PMC6411286.

43. Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengström Y, Sundberg CJ, Rundqvist H. 2018; Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 32:5495–5505. DOI:
10.1096/fj.201700968R. PMID:
29750574.

44. Paolini A, Omairi S, Mitchell R, Vaughan D, Matsakas A, Vaiyapuri S, Ricketts T, Rubinsztein DC, Patel K. 2018; Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci Rep. 8:9062. DOI:
10.1038/s41598-018-27429-7. PMID:
29899362. PMCID:
PMC5998118.

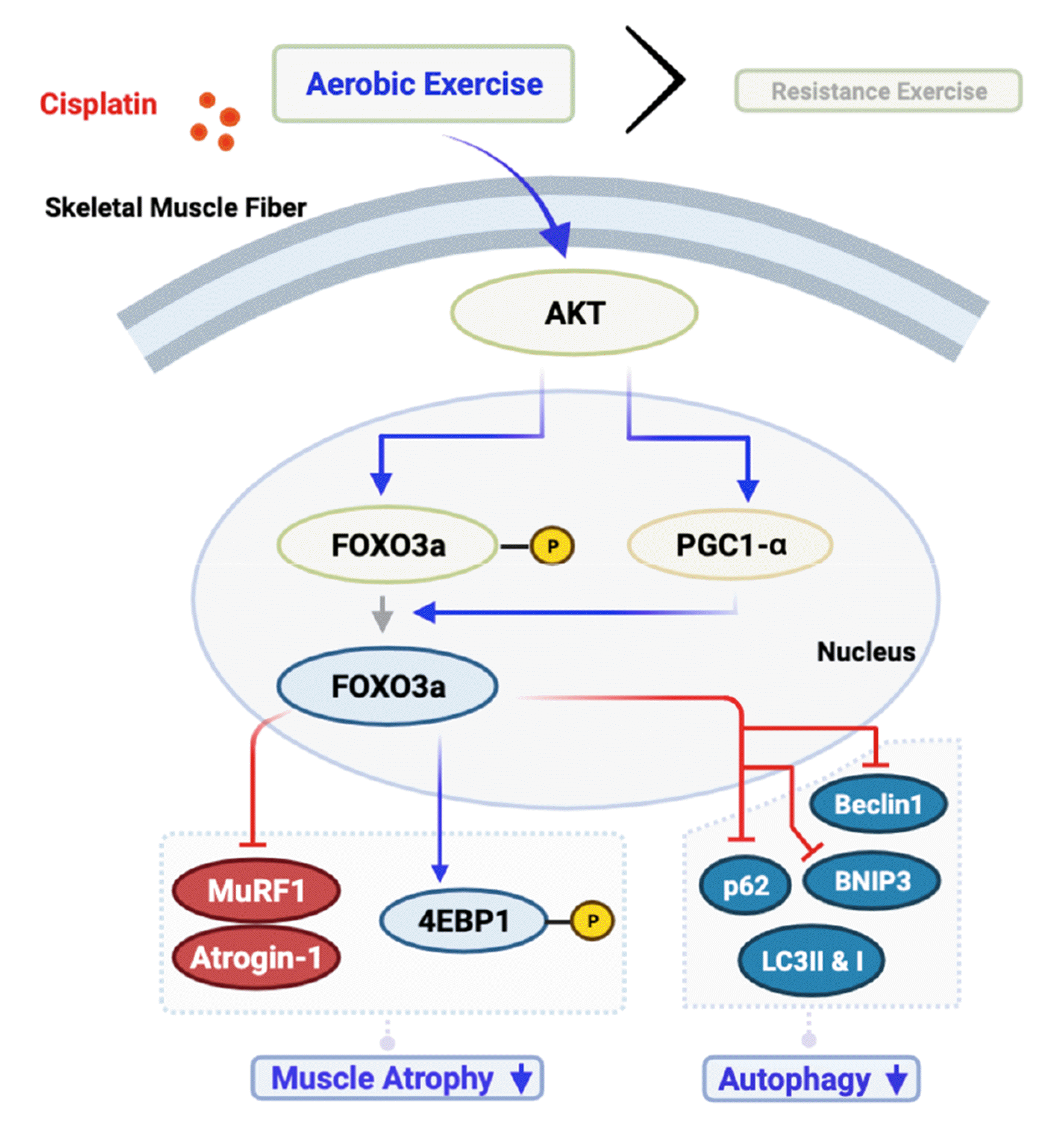




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download